O grafite, como um alótropo do carbono, aparece com frequência na vida cotidiana e na produção industrial. O que é grafite? É um metal, mineral ou elemento? Do que ele é feito? Este artigo explorará profundamente a natureza do grafite, revelando suas camadas de segredos. Além disso, mostrará o charme único e o importante valor do grafite no campo da ciência e das aplicações.
Índice
AlternarDefinição de grafite
O grafite é uma forma cristalina de carbono, geralmente apresentando um estado sólido cinza-preto e opaco, com um brilho metálico único. Mas, às vezes, ele tem outra forma: a forma amorfa, que consiste em arranjos irregulares de átomos de grafite. Sua textura é relativamente macia, e essa maciez permite que ele deixe marcas claras no papel. Essa propriedade também faz com que o grafite se torne o principal componente da grafite do lápis. Na essência química, o grafite pertence ao alótropo do carbono, que é composto de átomos de carbono como o diamante, o fulereno e outras substâncias. No entanto, a maneira como os átomos de carbono estão dispostos nessas substâncias é muito diferente, resultando em enormes diferenças em suas propriedades físicas e químicas.
O grafite é um metal ou um mineral?
O grafite não é um metal, mas um mineral. O metal geralmente tem boa condutividade elétrica, condutividade térmica, ductilidade e outras características típicas. Embora o grafite tenha um certo grau de condutividade elétrica e térmica, ele não tem a ductilidade característica dos metais. O grafite é um produto de ocorrência natural de processos geológicos complexos e atende à definição de mineral. Ele existe em rochas e depósitos específicos na natureza. E é uma forma importante de carbono no longo ciclo e no processo de evolução do círculo terrestre, testemunhando o movimento e a mudança de materiais no interior da Terra.
O grafite é um elemento?
O grafite não é um elemento, mas uma substância única composta de carbono. Um elemento é um grupo de átomos que tem o mesmo número de cargas nucleares (prótons). O grafite é uma entidade material formada por um grande número de átomos de carbono unidos por ligações químicas específicas. Essa substância pura composta pelos mesmos elementos é chamada de elementar. O grafite é uma forma especial de existência elementar do carbono. Com uma estrutura cristalina e propriedades físicas e químicas exclusivas, ele mostra as propriedades ricas e diversas do carbono.
O grafite é carbono?
O grafite é um alótropo do carbono, composto inteiramente pelo elemento carbono. Dentro do grafite, os átomos de carbono estão dispostos e combinados de uma maneira muito especial para formar uma estrutura cristalina exclusiva. Essa estrutura confere ao grafite muitas propriedades especiais. Assim, ele e outros alótropos de carbono, como o diamante (conhecido por suas propriedades duras e transparentes) e o fulereno (com uma estrutura esférica ou tubular exclusiva), têm aparência e propriedades físicas e químicas significativamente diferentes. Em muitos campos, eles desempenham papéis diferentes.
De onde vem o grafite?
Fontes naturais
As fontes naturais de grafite na natureza são mais extensas. Podemos formar parte do grafite em rochas metamórficas. Por exemplo, no processo de metamorfismo regional, os sedimentos originais que contêm carbono (como veios de carvão) são gradualmente transformados em grafite por meio de uma complexa cristalização metamórfica sob condições extremas de alta temperatura e pressão. Além disso, parte do grafite vem de rochas magmáticas. E quando o magma invade a crosta, o carbono contido no magma se cristaliza em um ambiente geológico específico e em condições físicas e químicas. Assim, forma-se o grafite. Os depósitos naturais de grafite estão distribuídos em muitos países e regiões do mundo, entre os quais a China, o Brasil, a Índia e outros países têm recursos naturais de grafite relativamente ricos. Esses recursos fornecem uma base material importante para o desenvolvimento de setores globais relacionados ao grafite.
Grafite artificial
Com o rápido desenvolvimento da tecnologia industrial moderna, a produção de grafite artificial tornou-se gradualmente uma parte importante do suprimento de grafite. O grafite artificial geralmente é produzido pelo tratamento térmico de alta temperatura de matérias-primas específicas que contêm carbono (como coque de petróleo, coque de asfalto etc.). Ele converte amorfo carbono em grafite. Em altas temperaturas, os elementos que não são carbono nessas matérias-primas contendo carbono se volatilizarão gradualmente. E os átomos de carbono se reorganizarão e se cristalizarão, formando o grafite artificial com uma estrutura semelhante à do grafite natural. A produção de grafite artificial tem um alto grau de controle. E pode controlar com precisão sua pureza, estrutura cristalina e propriedades físicas e químicas de acordo com as diferentes necessidades de aplicação industrial. Por isso, é amplamente utilizado em muitos campos, como aço, baterias, refratários e assim por diante. Isso proporciona um forte suporte para o desenvolvimento da indústria moderna.
Tipos de grafite
Em resumo, o grafite pode ser dividido principalmente em dois tipos: grafite natural e grafite sintético.
Grafite natural
O grafite natural também inclui o grafite em flocos, o grafite cristalino e o grafite criptocristalino.
O grafite em flocos é caracterizado por uma forma de flocos grandes e finos com uma ampla faixa de diâmetro, variando de alguns décimos de milímetro a vários milímetros. Essas escamas têm boa condutividade elétrica e térmica na direção plana, enquanto sua estrutura em camadas lhe confere excelentes propriedades de lubrificação.
Os cristais de grafite cristalino são bem desenvolvidos, mostrando uma óbvia forma de cristal lamelar hexagonal. Suas escalas são relativamente grandes, e o diâmetro das fatias costuma ser superior a 0,1-0,2 mm. Esse tipo de grafite não é abundante na natureza. Porém, devido à sua excelente estrutura cristalina e propriedades exclusivas, ele desempenha um papel importante em muitos campos industriais de ponta.
O cristal de grafite afanítico é muito pequeno e existe na forma de agregados microcristalinos. E a forma específica do cristal dificilmente pode ser distinguida a olho nu. Seu conteúdo de carbono fixo está em um nível alto, aproximadamente na faixa de 60%-80%. Em aplicações industriais, podemos usar o grafite criptocristalino como material de fundição e refratário.
Grafite sintético
O grafite sintético contém três tipos, cada um com propriedades e usos exclusivos.
A pureza do grafite de alta pureza é extremamente alta, o teor de impurezas é muito baixo, geralmente superior a 99,9%. Sua excelente estabilidade química e alta condutividade elétrica fazem com que ele desempenhe um papel fundamental na fabricação de semicondutores, na indústria química de ponta e em outros setores com requisitos rigorosos de pureza.
O grafite prensado isostaticamente é formado pelo processo de prensagem isostática e tem as características de estrutura uniforme e isotropia. Tem excelente resistência mecânica, resistência a altas temperaturas e resistência a choques térmicos, excelente desempenho em metalurgia, EDM e outros campos.
O grafite expandido é feito de grafite natural por meio de um tratamento especial, com uma estrutura sem fim exclusiva. Ele pode se expandir rapidamente em altas temperaturas para formar um material com excelentes propriedades de isolamento térmico e vedação. No campo dos materiais à prova de fogo, o grafite expandido pode ser usado para fabricar vedações à prova de fogo, revestimentos à prova de fogo, etc. Ele pode impedir efetivamente a propagação do fogo.
De que é feito o grafite?
Elemento de grafite
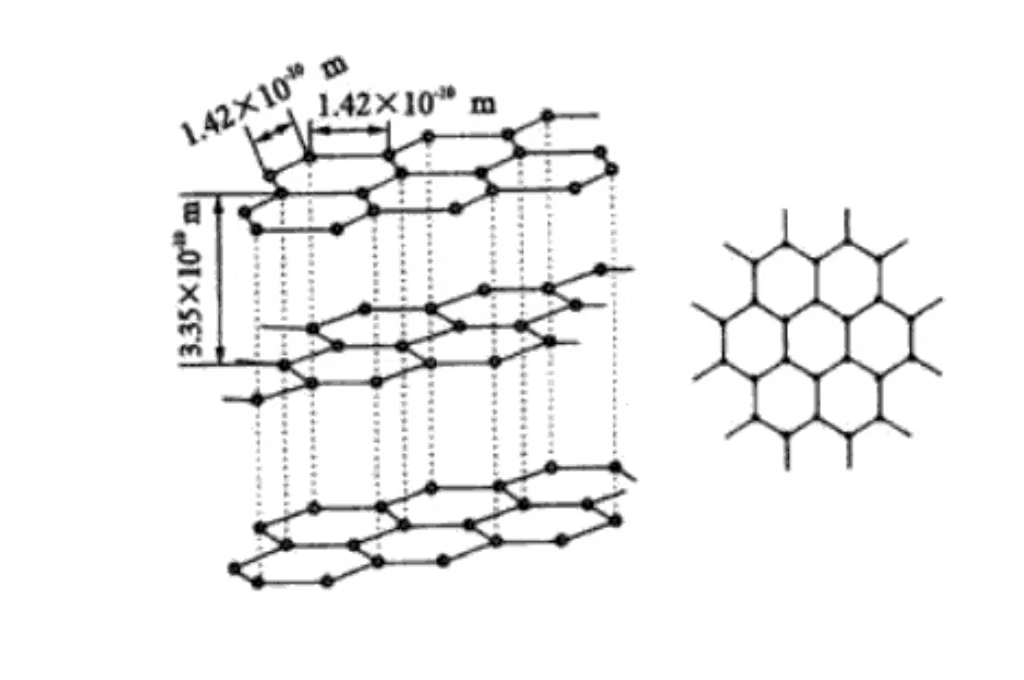
O principal elemento constituinte do grafite é o carbono. No grafite, os átomos de carbono são conectados uns aos outros por ligações covalentes, formando uma rede planar de hexágonos. Essas estruturas de rede planar são empilhadas camada sobre camada no espaço, formando a estrutura cristalina exclusiva do grafite. Cada átomo de carbono forma uma ligação covalente com três átomos de carbono ao redor. E a presença dessa ligação covalente confere ao grafite alta estabilidade e resistência dentro da camada. Ao mesmo tempo, estabelece a base para algumas de suas propriedades especiais, como a condutividade elétrica e térmica.
A fórmula química do grafite
A fórmula química do grafite é geralmente denotada por C, o que indica claramente que ele é composto inteiramente de carbono. Apesar de sua fórmula química simples, o grafite apresenta propriedades físicas e químicas complexas e diversas. Isso se deve ao arranjo exclusivo dos átomos de carbono e à existência de muitas formas de ligação química. Essa característica, que é composta de um único elemento, mas tem propriedades ricas, faz com que o grafite ocupe uma posição única no campo da ciência dos materiais. E também faz com que ele se torne o foco de inúmeras pesquisas e usos.
Estrutura de grafite
Carbono Disposição dos átomos de carbono no grafite
O carbono está disposto na microestrutura do grafite. E os átomos de carbono apresentam uma característica notável de disposição em camadas. Cada camada de átomos de carbono está intimamente organizada para formar uma enorme estrutura de rede planar hexagonal. E os átomos de carbono estão intimamente conectados por meio de ligações covalentes. Isso faz com que essas camadas planas tenham alta estabilidade e resistência e possam suportar um certo grau de força externa sem sofrer danos. A camada entre as camadas se dá por meio da fraca interação da força de van der Waals. Essa força relativamente fraca entre as camadas faz com que o grafite entre a camada e a camada quando a pequena força externa é fácil de deslizar, o que proporciona ao grafite boa lubrificação e flexibilidade. Portanto, ele precisa reduzir o atrito e ter uma certa capacidade de deformação no cenário de aplicação.
Ligação
A ligação covalente entre os átomos de carbono na camada de grafite é uma ligação química forte. Ela não apenas garante a estabilidade e a integridade da camada de grafite, mas também tem um impacto profundo nas propriedades físicas do grafite. Devido à existência de ligações covalentes, os elétrons podem se mover com relativa liberdade entre os átomos de carbono dentro da camada. Isso faz com que o grafite tenha boa condutividade térmica e condutiva dentro da camada e possa transferir corrente e calor com eficiência.
A força de van der Waals entre as camadas é relativamente fraca. E sua contribuição para as propriedades físicas do grafite, como dureza e densidade, é menor do que a das ligações covalentes. Esse efeito sinérgico das ligações covalentes intra-camadas e das forças de van der Waals inter-camadas cria as propriedades anisotrópicas exclusivas do grafite. Ou seja, as propriedades físicas e químicas do grafite na direção da camada e da camada vertical são significativamente diferentes. E essa propriedade precisa ser totalmente considerada nas aplicações de materiais para obter a utilização ideal das propriedades do grafite.
Propriedades do grafite
Propriedades físicas
Cor
O grafite geralmente apresenta uma cor de aparência cinza-preta. A formação dessa cor está intimamente relacionada à estrutura eletrônica interna do grafite e às suas características de absorção e reflexão da luz. Os átomos de carbono no grafite absorvem e dispersam a luz visível por meio de ligações químicas específicas e distribuição de nuvens de elétrons. Assim, a maior parte da luz visível é absorvida, e apenas uma pequena quantidade de luz é refletida ou espalhada. Assim, apresenta um efeito visual cinza-escuro em nível macro. Além disso, a natureza opaca do grafite também confere à sua aparência uma textura exclusiva, em nítido contraste com outros materiais transparentes ou translúcidos.
Densidade
A densidade do grafite é relativamente pequena, entre 2,09 e 2,23 g/cm 3. E a gravidade específica também é baixa. Essa característica torna o grafite uma clara vantagem em alguns cenários de aplicação com requisitos rigorosos de peso. Por exemplo, no projeto de determinados componentes no campo aeroespacial, se for necessário usar materiais que tenham um certo grau de propriedade condutora e lubrificação, mas que também possam reduzir o peso total. Então, o grafite se torna um material candidato muito potencial.
Ponto de fusão
O grafite tem um ponto de fusão muito alto, em torno de 3652°C -3697 °C. Essa excelente estabilidade em altas temperaturas permite que o grafite mantenha sua estrutura e propriedades relativamente estáveis em ambientes com temperaturas extremamente altas. Na fundição de ferro e aço, em materiais refratários e em outros processos industriais de alta temperatura, o grafite desempenha um papel fundamental.
Condutividade elétrica
O grafite tem excelente condutividade elétrica na camada, o que se deve à formação de uma estrutura estável de nuvem de elétrons entre os átomos de carbono na camada por meio de ligações covalentes. E os elétrons podem se mover com relativa liberdade nessa estrutura, de modo a obter uma condução eficiente da corrente. Ao mesmo tempo, a condutividade térmica do grafite também é muito boa, e ele pode transferir calor rapidamente.
Lubrificação
As propriedades de lubrificação do grafite lubrificante se devem à sua estrutura exclusiva em camadas. Como a força de van der Waals entre as camadas é fraca. Quando o grafite é submetido a uma força externa, o deslizamento relativo entre as camadas é fácil de ocorrer. E esse processo de deslizamento pode reduzir efetivamente o coeficiente de atrito, de modo a desempenhar um bom papel de lubrificação. Seja na manutenção diária de lubrificação de vários equipamentos mecânicos no setor de maquinário ou nas necessidades de lubrificação em alguns ambientes especiais (como alta temperatura, alta pressão ou ambiente de corrosão química), o grafite pode apresentar excelentes efeitos de lubrificação.
Propriedades químicas
Resistência à corrosão do grafite
O grafite tem boa resistência a ácidos e álcalis. Ele pode manter a estrutura e o desempenho relativamente estáveis em soluções ácidas e alcalinas em uma determinada faixa de concentração. Isso ocorre porque os átomos de carbono no grafite formam uma estrutura de energia de ligação química estável por meio de ligações covalentes. Isso faz com que o grafite seja difícil de ser destruído por íons em soluções ácidas e básicas. Essa resistência a ácidos e álcalis faz com que o grafite tenha um valor de aplicação importante em alguns ambientes corrosivos no setor químico.
Reatividade com outros materiais
Em condições normais de temperatura, as propriedades químicas do grafite são relativamente estáveis. E não é fácil reagir quimicamente com a maioria das substâncias comuns. Entretanto, quando exposto a alta temperatura, alta pressão ou a um ambiente químico específico, o grafite pode reagir com alguns oxidantes (como oxigênio, ácido sulfúrico concentrado, etc.).
Por exemplo, quando o oxigênio é suficiente e a temperatura aumenta até certo ponto, o grafite sofre uma reação de oxidação e se transforma gradualmente em produtos como o dióxido de carbono. Essa reatividade limita, até certo ponto, a aplicação do grafite em alguns ambientes de oxidação extrema. Mas ela também oferece a possibilidade de alguns tratamentos e modificações especiais do grafite.
Coeficiente de expansão térmica
O grafite tem um baixo coeficiente de expansão térmica, o que lhe confere boa estabilidade dimensional quando a temperatura muda. Em comparação com muitos outros materiais, o volume do grafite muda muito pouco durante o processo de aumento e queda de temperatura.
Em algumas aplicações que exigem alta precisão dimensional dos materiais, esse baixo coeficiente de expansão térmica do grafite é particularmente importante. Ele pode efetivamente evitar problemas como a deformação de componentes e a redução da precisão da montagem causada por flutuações de temperatura. Assim, garante a operação normal e o desempenho estável de equipamentos ou instrumentos em diferentes ambientes de temperatura.
Oxidação
Embora o grafite apresente forte resistência à oxidação e à corrosão em temperatura ambiente, sob condições extremas, como alta temperatura, alta umidade ou ambiente de forte oxidação, o grafite sofrerá oxidação e corrosão gradualmente. Por exemplo, com a exposição prolongada a altas temperaturas no ar, os átomos de carbono na superfície do grafite reagirão com o oxigênio para formar uma camada de óxido.
E, com o tempo, o espessamento contínuo da camada de óxido levará a mudanças na estrutura e no desempenho do grafite. Por exemplo, a redução da condutividade e da resistência. Portanto, em algumas áreas de aplicação com altos requisitos de resistência à oxidação do grafite, muitas vezes é necessário realizar um tratamento especial da superfície do grafite. Ou adicionar antioxidantes e outras medidas. Para melhorar sua capacidade antioxidante e garantir a estabilidade do desempenho e a confiabilidade dos materiais de grafite durante o uso.
Propriedades mecânicas
Dureza e resistência
A resistência e a dureza do grafite são relativamente baixas, e sua dureza Mohs é de aproximadamente 1-2. Essa propriedade torna o grafite relativamente fácil de moldar e processar em vários formatos durante o processamento. Por exemplo, na fabricação de ponteiras de lápis, ao misturar grafite e outros materiais, como argila, em diferentes proporções e pressionar, é conveniente fazer ponteiras de caneta com diferentes níveis de dureza para atender a diferentes necessidades de escrita. Entretanto, embora a resistência geral do grafite seja baixa, ele ainda tem um certo valor de utilização de resistência em algumas direções específicas. Por exemplo, ao longo da direção da camada de grafite.
Elasticidade
Devido à estrutura única em camadas do grafite, ele apresenta flexibilidade e elasticidade até certo ponto. Quando submetido a uma pequena força externa, podemos dobrar e deformar a estrutura em camadas do grafite até certo ponto. E quando a força externa é removida, podemos restaurar o grafite à sua forma original ou próxima da forma original. Essa flexibilidade e elasticidade tornam o grafite um potencial de aplicação em alguns dispositivos eletrônicos flexíveis, materiais de vedação e outros campos emergentes.
Anisotropia
O grafite anisotrópico tem características anisotrópicas extremamente óbvias. Ou seja, suas propriedades físicas e químicas são significativamente diferentes em diferentes direções. Em termos de propriedade condutora, a condutividade ao longo da camada de grafite é muito maior do que a condutividade na camada vertical. Isso é causado pela promoção de ligações covalentes na condução de elétrons e pelo impedimento da força de van der Waals entre camadas na condução de elétrons.
Em termos de dureza e resistência, a dureza e a resistência da camada vertical são relativamente altas. Como a força de van der Waals entre as camadas limita o deslizamento relativo entre as camadas até certo ponto. Já o deslizamento e a deformação têm maior probabilidade de ocorrer devido à fraca força entre as camadas. Essa característica anisotrópica requer atenção e consideração especiais no processo de aplicação do grafite. De acordo com os requisitos específicos da aplicação, é razoável selecionar e utilizar as vantagens das propriedades do grafite em diferentes direções. Assim, é possível maximizar a utilização do desempenho do material de grafite e otimizar o efeito da aplicação.
Propriedades térmicas e elétricas
Há uma estreita relação interna entre as propriedades térmicas e elétricas do grafite e as propriedades térmicas e o excelente desempenho. Sua alta condutividade térmica é suficiente para emitir calor rapidamente, o que é de grande valor de aplicação no campo da dissipação de calor de equipamentos eletrônicos. Ao mesmo tempo, a boa condutividade do grafite permite que ele transmita a corrente de forma eficiente como um excelente condutor no circuito.
Outras propriedades
Além das muitas propriedades mencionadas acima, o grafite tem algumas outras propriedades especiais. Por exemplo, o grafite tem certas propriedades de adsorção. Sua rica estrutura de poros e a grande área de superfície específica podem absorver alguns gases e pequenas moléculas. Esse recurso tem valor potencial de aplicação na purificação de gás e no tratamento de esgoto no campo da proteção ambiental. Com a modificação e o tratamento adequados do grafite, seu desempenho de adsorção pode ser melhorado ainda mais. Ele pode ser usado para remover gases nocivos no ar (como formaldeído, dióxido de enxofre, etc.) ou íons de metais pesados na água, poluentes orgânicos, etc.
6 Usos do grafite
Em Lápis
O grafite é um componente essencial da grafite de lápis. Devido à sua textura macia e estrutura em camadas exclusiva, ele pode deixar marcas nítidas no papel após a mistura e o ajuste da dureza com argila para atender às necessidades de escrita e pintura. Do aprendizado do aluno à criação artística, ela é amplamente utilizada em todos os tipos de ferramentas de escrita. Isso permite que as pessoas expressem livremente suas ideias e criatividade.
Como lubrificante
O grafite como lubrificante tem boa lubricidade devido à fraca força de van der Waals na camada intermediária e na camada intermediária da estrutura em camadas. Ele é amplamente utilizado no campo mecânico. Seja nas peças móveis internas do motor de automóveis ou nas peças de transmissão mecânica industrial. Ou mesmo nas peças aeroespaciais de alta temperatura e alta pressão, o pó de grafite pode efetivamente reduzir o atrito e o desgaste. Além disso, ele garante a operação suave e eficiente do equipamento e prolonga a vida útil.
Fabricação de aço
O grafite desempenha um papel importante na produção de aço. Como um eletrodoO sistema de carburação de aço é um sistema de fusão por calor de Joule, que pode introduzir corrente para produzir a fusão por calor de Joule de matérias-primas de sucata de aço. Como um carburador, o teor de carbono do aço fundido pode ser ajustado com precisão. Feito de material de revestimento do forno, em virtude da resistência a altas temperaturas e à corrosão, protege o corpo do forno de fabricação de aço contra danos causados pelo aço fundido em alta temperatura e pela escória. Além disso, o grafite tem uma certa capacidade térmica específica. Ele pode absorver e liberar calor durante o processo de fabricação de aço, ajudando a regular as flutuações de temperatura no forno. Ele apoia de forma eficaz o desenvolvimento eficiente, seguro e estável do processo de fabricação de aço.
Bateria
O grafite é de grande importância em baterias e é comumente usado como material de eletrodo negativo em baterias de íons de lítio baterias. Sua estrutura em camadas oferece espaço para a incorporação e desincorporação de íons de lítio, incorporação de carga, descarga de descarga, com boa propriedade condutora. Isso garante o ciclo de carga e descarga da bateria. Na pesquisa da tecnologia de baterias emergentes, ele também é considerado o material básico. Isso desempenha um papel importante na promoção do desenvolvimento de um novo armazenamento de energia.
Materiais refratários
O grafite tem alto ponto de fusão e estabilidade em altas temperaturas, além de ser um material refratário de alta qualidade. Em metalurgia, cerâmica, vidro e outros processos industriais de alta temperatura, é usado na fabricação de tijolos refratários, revestimentos cadinhos e assim por diante. Ele pode resistir à erosão do metal derretido e da escória em ambientes de alta temperatura e manter a estabilidade estrutural. Reduz a perda de calor, reduz o risco de acidentes e constrói uma forte linha de segurança para a produção industrial em alta temperatura.
Reatores nucleares
O grafite serve como moderador de nêutrons em reatores nucleares. Ao colidir com nêutrons, os nêutrons rápidos são desacelerados para nêutrons térmicos. Assim, eles controlam a taxa de reação de fissão nuclear e garantem a operação estável do reator. Os primeiros reatores nucleares têm muitos usos. Mas o grafite se altera sob alta temperatura e irradiação de nêutrons, exigindo respostas técnicas especiais para garantir o uso seguro a longo prazo.
Conclusão
Como um alótropo do carbono, o grafite tem várias propriedades e é amplamente utilizado. Suas várias características estão relacionadas entre si, o que determina o desempenho em diferentes cenários. Desde lápis de uso diário até a fabricação de aço industrial e de baterias e reatores nucleares de alta tecnologia, o grafite é indispensável. Com o desenvolvimento da ciência e da tecnologia, o grafite tem grande potencial em campos emergentes. E ocupará uma posição mais crítica na ciência dos materiais, na estratégia de recursos globais e na estratégia de desenvolvimento sustentável. E continuará a promover o progresso da sociedade humana.