Meskipun keduanya sama-sama karbon, grafit dan graphene tidak bisa lebih berbeda. Keduanya memiliki serangkaian karakteristik khusus yang memungkinkannya untuk digunakan di berbagai bidang. Dalam artikel ini, Anda akan belajar tentang struktur dan penggunaan & biaya. Mari kita selami.
Daftar Isi
Beralih
Grafit vs Graphene
Apa itu Grafit
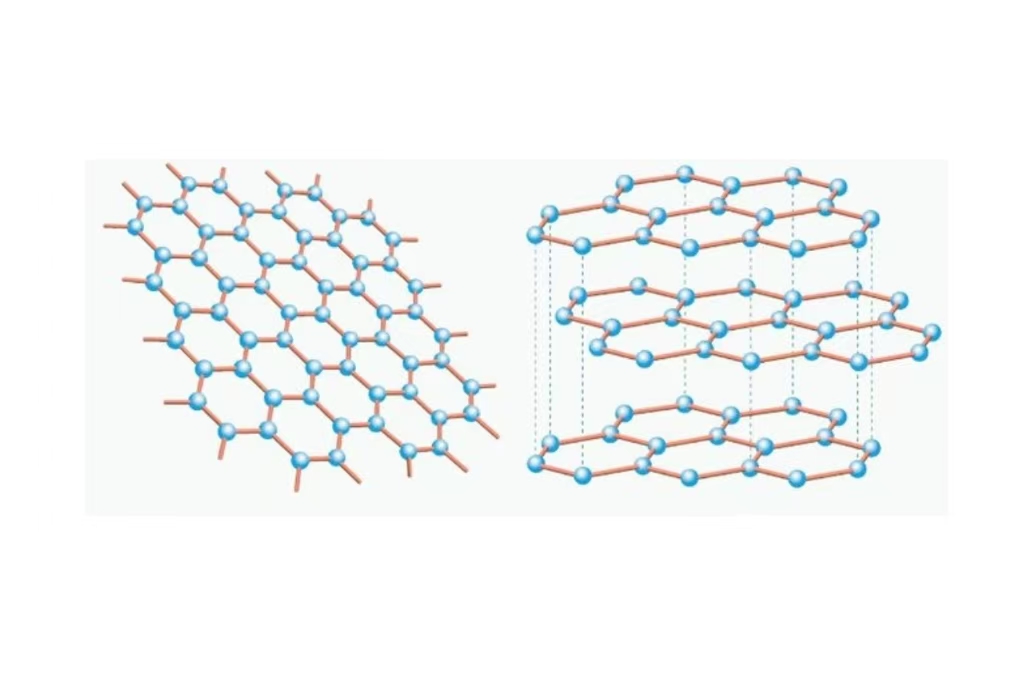
Lembaran grafit (alotrop karbon) dari karbon adalah kisi-kisi heksagonal. Rasa pelumas yang dimiliki grafit diambil dari kemampuan lapisannya untuk meluncur satu sama lain. Grafit sekarang ditemukan dalam barang sehari-hari, dari pensil hingga pelumas dan bahkan baterai. Grafit masih merupakan konduktor (meskipun tidak sekonduktif graphene, karena berlapis-lapis). Murah dan relatif berlimpah, ini adalah kandidat utama untuk banyak penggunaan industri.
Apa itu Graphene
Graphene adalah susunan sarang lebah atom karbon dalam satu lapisan. Ketebalannya juga hanya satu atom, jadi itu berarti materialnya bisa sangat tipis dan masih sangat kuat. Graphene sering disebut sebagai "bahan ajaib" karena ringan dan nyaris transparan, namun juga karena lebih kuat dari apa pun yang pernah kita temui dengan konduktivitas listrik yang baik. Bahan ini 200 kali lebih kuat dari baja, dan setebal satu atom. Aplikasinya sangat banyak, dari elektronik hingga peralatan medis, dan meskipun bahan ini relatif melimpah di alam, namun pembuatannya sangat mahal dan sulit.
Struktur
Lapisan vs Lapisan Tunggal
Grafit: Terdiri dari banyak lapisan lembaran karbon yang ditumpuk satu sama lain. Karena lapisan-lapisan ini saling mengikat satu sama lain secara lemah, maka lapisan-lapisan ini dapat dengan mudah bergeser satu sama lain. Inilah alasan mengapa kita merasakan grafit di tangan kita terasa licin dan juga berperilaku seperti pelumas.
Graphene: Lapisan sarang lebah tunggal dari atom karbon, bahan setebal satu atom dengan kekuatan dan fleksibilitas yang luar biasa.
Perbedaan Ikatan
Grafit: Karena lapisan-lapisannya disatukan oleh gaya van der Waals primitif, grafit mudah terbelah atau terbelah.
Graphene: Atom-atom karbon ini terikat sangat erat di dalam lapisan tunggal, yang mengilhami graphene dengan kekuatan dan fleksibilitasnya yang luar biasa.
Sifat-sifat Grafit dan Grafena
Konduktivitas Listrik
Grafit: Meskipun grafit juga mentransfer listrik berkat elektron yang bergerak bebas di antara lapisan-lapisannya, namun grafit yang tidak memiliki lembaran graphene tambahan di sini tidak begitu konduktif. Hambatan muncul ketika lapisan-lapisan tersebut dipisahkan oleh jarak yang cukup jauh.
Graphene: Salah satu konduktor listrik yang paling terkenal Karena elektron dapat melewatinya tanpa hambatan, graphene adalah bahan alami untuk elektronik berkecepatan tinggi.
Kekuatan Mekanis
Grafit: Lembut dan rapuh. Grafit mudah patah, oleh karena itu digunakan untuk ujung pensil. Mudah rapuh karena lapisan-lapisannya hanya disatukan oleh ikatan yang lemah.
Graphene: Sangat kuat, 20 kali lebih kuat dari baja. Meskipun hanya memiliki ketebalan satu atom karbon, struktur graphene disebabkan oleh kisi kristalnya yang kuat dan karena setiap ikatan C-C memiliki kekuatan yang setara dengan dua kali lipat.
4.3 Konduktivitas Termal
Grafit: Konduktivitas termal yang baik, sehingga dapat digunakan secara luas, misalnya dalam pendinginan pasif.
Graphene: Konduktivitas termal yang luar biasa. Graphene adalah salah satu konduktor terbaik untuk energi panas, sehingga menjadi pilihan yang baik untuk mendinginkan komponen listrik.
Aplikasi
Grafit
Pensil: Pensil menggunakan grafit sebagai "timah" karena tidak akan terlalu banyak mengotori atau menodai, dan akan menandai permukaan dengan mudah dan kemudian menghapusnya apabila diperlukan. Grafit terdiri dari lembaran-lembaran dalam satu lapisan, sehingga dapat menulis dengan sedikit tekanan dan meninggalkan bekas di atas kertas.
Pelumas: Karena licin, maka termasuk dalam pelumas untuk mencegah gesekan di antara mesin. Karena lapisan grafitnya licin, mereka memungkinkannya meluncur dengan mudah di atas satu sama lain sehingga menciptakan penghalang yang kokoh yang mengurangi keausan komponen akibat gerakan.
Baterai: Grafit juga ditemukan sebagai elemen intrinsik yang dapat melayani dua fungsi utama dalam anoda baterai lithium-ion; stabilitas dengan penyimpanan energi yang efisien. Struktur berlapis ini memungkinkan ion lithium untuk dengan mudah mengalir masuk dan keluar selama siklus pengisian/pengosongan.
Pembuatan baja: Selama pembuatan baja, umumnya digunakan sebagai bahan tahan api karena mampu menahan panas tingkat tinggi. Untuk melakukan ini, isolasi pelapis dalam pengecoran dan tungku pelapisan harus mempertahankan suhu yang konstan - persyaratan mutlak untuk pembuatan baja berkualitas tinggi.
Grafena
Elektronik: Karena graphene adalah konduktor listrik yang sangat baik, satu kemungkinan yang disarankan oleh dua eksperimen baru di Berkeley Lab dan UC San Diego adalah menggunakannya sebagai pengganti silikon pada chip komputer - memungkinkan perangkat yang lebih cepat yang bekerja lebih dingin namun (idealnya) mengkonsumsi lebih sedikit energi per komputasi. Mobilitas elektronnya yang tinggi membuatnya cocok untuk kecepatan perpindahan cepat yang dibutuhkan dalam teknologi komputasi masa depan.
Perangkat Medis: Biokompatibilitas dan grafik yang sensitif membuatnya ideal untuk biosensor, sistem pengiriman obat serta rekayasa jaringan, dll. Non-toksisitas dan kompatibilitas dengan sistem biologis ini membuatnya menarik untuk digunakan dalam diagnostik medis atau perawatan terapeutik.
Penyimpanan Energi: Superkapasitor dan baterai canggih dapat menggunakan graphene di masa depan untuk memungkinkan waktu pengisian daya yang lebih cepat dengan penyimpanan energi yang lebih tinggi. Karena luas permukaan dan konduktivitasnya yang tinggi, kinerja perangkat penyimpanan energi dapat ditingkatkan, membuatnya tahan lama.
Material Komposit: Graphene dapat dikombinasikan dengan bahan lain seperti logam dan plastik untuk membuatnya lebih ringan sekaligus lebih kuat atau konduktif secara elektrik. Aplikasi potensial dari komposit yang diperkuat graphene ini adalah untuk mengurangi berat badan, meningkatkan efisiensi bahan bakar dan meningkatkan kinerja ablasi secara keseluruhan.
Grafit vs Graphene: Biaya
Grafit: Rendah
Berlimpah: Grafit tersedia dengan harga murah dalam bentuk alami yang umum dan ditambang di seluruh dunia, sehingga merupakan mineral yang mudah didapat dan hemat biaya.
Biaya Produksi: Karena biaya produksi dan pemrosesan yang rendah, grafit juga cocok untuk digunakan dalam banyak aplikasi industri.
Graphene: Tinggi
Tantangan Produksi: Memproduksi graphene jauh lebih sulit daripada memproduksi grafit. Meskipun monolayer h-BN sekarang dapat dibuat dengan menggunakan metode basah, pendekatan fabrikasi yang ada - seperti deposisi uap kimia (CVD) dan teknik pengelupasan kulit. Pendekatan ini tetap mahal dan tidak mudah diskalakan untuk manufaktur skala besar.
Tren Harga: Meskipun ada tren penurunan harga graphene, biaya untuk bahan ini jauh lebih tinggi daripada grafit. Saat ini sedang dilakukan penelitian untuk menciptakan cara yang lebih hemat biaya dalam memproduksi graphene sehingga bahan ini menjadi layak secara komersial.
Kesimpulan
Grafit dan graphene memiliki beberapa sifat unik yang mirip satu sama lain yang membuatnya menjadi kelompok material yang penting. Oleh karena itu, kita dapat menganggap graphene sebagai langkah terakhir dalam evolusi karbon. Grafit telah melayani industri selama beberapa dekade, tetapi Graphene adalah apa yang banyak orang percaya sebagai pengubah permainan yang nyata yang dapat mengganggu banyak bidang di berbagai sektor. Jika orang dapat memproduksi graphene dengan lebih murah dan sederhana karena penelitian saat ini, dunia di mana material dunia lain menjadi raja mungkin tidak akan terlalu jauh. Apakah Anda dapat menghormati pragmatisme grafit atau tergoda oleh potensi graphene sekarang. Keduanya harus bekerja sama untuk bergerak maju dalam teknologi dan industri.