Introduction
Pourquoi le graphite est-il spécial ? Il est solide et glissant. Il déplace facilement l'électricité. Il résiste à la corrosion à haute température. Vous en apprendrez plus sur les propriétés du graphite ici. Nous allons examiner de plus près ce qui fait du graphite un matériau si parfait pour les machines. Si vous voulez savoir comment le graphite aide les ingénieurs, continuez à lire.
Table des matières
Toggle
Comprendre les propriétés physiques du graphite !
Le graphite est constitué de carbone. Les couches sont souples et se déplacent facilement. Chaque couche est plate. Les propriétés du graphite comprennent sa légèreté, environ 2,26 g/cm³. On peut le toucher et il est glissant. C'est ainsi qu'il est utilisé pour faire circuler l'électricité. Le graphite est également utilisé dans les piles et les crayons.
Non seulement il ne fond qu'à 3550°C, mais il est également très résistant. C'est cette particularité qui le rend utile à l'intérieur des machines. Le graphite est différent du diamant, mais il est très utile. Le carbone peut également se présenter sous différentes nuances ; ils sont tous les deux, mais ils fonctionnent différemment.
Propriétés chimiques du graphite !
- Grande inertie
Graphite n'est pas facile à modifier. La plupart des produits chimiques ne changent pas. Les électrons qui se trouvent dans les atomes de carbone du graphite sont très puissants. Le graphite est sûr parce qu'ils sont disposés en couches. Il ne se corrode pas dans les acides ou les bases. En revanche, on peut utiliser le graphite dans les usines et il fonctionne à 3600°C !
C'est pourquoi il convient aux grandes machines. Il fonctionne à des températures très élevées et vous pouvez lui faire confiance. Dans les réacteurs nucléaires aussi, le graphite est solide. Vous constaterez qu'il dure plus longtemps que n'importe quel autre matériau. C'est ainsi que Jinsun Carbon fabrique des électrodes en graphite qui peuvent encore fonctionner dans les usines à haute température, pendant longtemps.
- Résistance à l'oxydation
Le graphite est résistant. Il ne se casse pas à chaud. En dessous de 600°C, il ne réagit pas à l'oxygène. Il est donc sans danger pour les usines. Il est utilisé dans les fours (si vous l'utilisiez pour créer des fours). électrodes).
Les propriétés du graphite incluent sa résistance à la chaleur. En effet, le graphite se protège contre la dégradation et dure plus longtemps. Dans les usines métallurgiques où la chaleur est très élevée, il est préférable de l'utiliser. Tout reste sûr et solide. Jinsun Carbon fournit des électrodes en graphite pour les usines métallurgiques qui supportent la chaleur extrême en toute sécurité.
- Résistance à l'acide
Le graphite ne craint pas les acides. Il ne réagit pas à un acide fort, comme l'acide sulfurique. On le trouve dans les piles ou dans les usines chimiques. Ce matériau permet de travailler dans des endroits difficiles.
Le graphite dure parce que les atomes sont fortement liés. Les propriétés du graphite font qu'il ne change pas, même dans l'acide. Il maintient les machines en état de marche. Dans de nombreuses industries où l'on peut briser d'autres matériaux, à l'exception d'un acide fort, on l'utilise.
- Résistance aux alcalins
Dans un milieu alcalin, le graphite reste solide. Il ne réagit pas aux produits chimiques puissants, comme l'hydroxyde de sodium. Il est difficile à décomposer. Vous avez besoin de choses qui durent dans votre usine.
Le graphite résiste aux produits chimiques qui pourraient endommager d'autres objets. Le graphite est sûr parce que les atomes de carbone le maintiennent ainsi. Il est très résistant aux alcalis et peut être utilisé là où d'autres matériaux ne durent pas longtemps. Il fonctionne lorsque les choses vont mal, mais aussi lorsqu'elles vont bien.
- Stabilité thermique
Le graphite est stable à la chaleur. Il peut supporter des températures allant jusqu'à 3600°C. Il ne fond pas. Mais les couches de carbone restent solides et sûres. Il peut être utilisé dans des machines très chaudes comme les boucliers thermiques et les pièces de fusées.
La chaleur est également présente dans le graphite. C'est pourquoi il est utilisé dans l'électronique. Il permet aux machines de rester froides. Sa stabilité thermique est l'une des meilleures propriétés du graphite. Il est présent dans de nombreux endroits où la chaleur est élevée, ce qui permet aux choses de mieux fonctionner. Ces conditions extrêmes ? Pas de problème, Jinsun Carbon's produits en graphite excel.
| Propriété | Graphite | Inertie | Résistance à l'oxydation | Résistance à l'acide | Résistance aux alcalins | Stabilité thermique |
| Niveau d'inertie | Haut | 9/10 | Moyen | Haut | Haut | Excellent |
| Temp. d'oxydation | > 600°C | N/A | Oui | Limitée | Oui | Jusqu'à 3000°C |
| Réaction acide | Résistant | Aucune réaction | Mineur | Stable | Aucune réaction | Stable |
| Réaction alcaline | Résistant | Aucune réaction | Oui | Oui | Stable | Stable |
| Conductivité thermique | 100-400 W/mK | Pas d'effet | Une certaine dégradation | Pas d'effet | Impact minimal | Reste élevé |
| Intégrité structurelle | Des liens solides | Maintenu | Pas de changement significatif | Aucun dommage | Maintenu | Reste intacte |
Tableau des propriétés chimiques du graphite !
Propriétés mécaniques et résistance du graphite !
- Faible résistance à la traction
L'arrachement du graphite n'est pas fort. Il peut se briser avec une force de 20 à 25 MPa. Lorsque l'on tire sur le graphite, il se casse car il ne peut pas supporter une telle force. Lorsqu'on l'étire, ses couches de carbone glissent. Au-delà de 500 MPa de résistance à la traction, les choses sont plus solides : l'acier, par exemple.
Lorsque vous réfléchissez aux propriétés du graphite, n'oubliez pas qu'il ne supporte pas bien l'étirement. Il se casse facilement si on le tire trop fort. La résistance à la traction correspond à la quantité de tension que l'on peut exercer sur un objet avant qu'il ne se brise.
- Résistance élevée à la compression
Si vous appuyez sur le graphite, il devient très résistant. Cette chose peut exercer une pression de 150 MPa sur vous. Il reste solide si vous appuyez dessus. Même sous l'effet d'une force importante, les couches de graphite sont difficiles à écraser. Le graphite est solide parce que les atomes hexagonaux l'aident à rester solide lorsqu'il est pressé.
Lorsque l'on examine les propriétés du graphite, on s'aperçoit qu'il supporte bien la pression. Sa solidité et sa résistance à la compression en font un bon candidat pour les joints qui doivent rester étanches.
- Comportement anisotrope
La façon dont vous poussez fait une différence pour le graphite. Il se déchire facilement dans un sens. Dans une autre, il résiste à la pression. Selon les ingénieurs, il s'agit d'un phénomène anisotrope.
Si on les sépare, les couches de carbone glissent l'une sur l'autre, mais résistent à la pression. Ces propriétés du graphite en font un matériau spécial pour des applications telles que électrodes. Il conduit même mieux l'électricité dans un sens que dans l'autre.
- Module d'élasticité
Le graphite se plie sous la pression, mais pas trop. Son module d'élasticité est de 10-15 GPa. La rigidité est ce que le module d'élasticité indique. Le graphite fléchit un peu lorsque l'on appuie dessus, mais il revient à la normale.
À titre d'exemple, l'acier est beaucoup plus rigide avec 200 GPa. Il est plus mou, car il l'est, mais il a néanmoins une bonne résistance. Son élasticité lui permet de se plier et de se rétracter.
- Résistance à la rupture
Si l'on pousse le graphite trop fort, il se fissure facilement. Sa résistance à la rupture est de 0,5 à 1,5 MPa-m¹/². Si l'on ne fait pas attention, on appuie dessus et il se fissure rapidement. Dès qu'une fissure commence, elle se propage.
Le graphite ne supporte pas bien les fissures, c'est pourquoi les ingénieurs doivent le traiter avec soin. Il résiste bien à la pression, mais il se brise si on le tire ou si on le frappe trop fort. En termes de résistance à la rupture, on sait jusqu'à quel point un matériau peut supporter un coup de poing avant de perdre sa forme.
| Propriété | Résistance à la traction | Résistance à la compression | Comportement anisotrope | Module d'élasticité | Résistance à la rupture |
| Unité | MPa | MPa | Variable (plans XY) | GPa | MPa-m^0.5 |
| Valeur | Faible (≈ 20-30 MPa) | Élevé (≈ 100-200 MPa) | Oui | Modéré (≈ 8-12 GPa) | Faible (≈ 1-2 MPa-m^0.5) |
| Directionnalité | Isotrope (faible) | Variable | Haut | Variable | Variable |
| Impact de l'application | Structures fragiles | Soutien structurel | Stabilité thermique | Limites de déformation | Résistance à la rupture |
| Effets de la température | Diminutions | Augmentations | Oui | Réduit | Réduit |
| Utilisation | Lubrifiants, joints | Matériaux réfractaires | Boucliers thermiques | Capteurs | Applications du stress |
Tableau sur les propriétés mécaniques et la résistance du graphite !
Conductivité électrique du graphite !
- Electrons libres
Le graphite possède des électrons libres. Trois des quatre atomes de carbone ont trois électrons qui se lient. Un électron se déplace librement. Cela permet à l'électricité de se déplacer. Il y a environ 6 x 10¹⁸ électrons libres dans un cm³ de graphite. Ils vont et viennent entre les couches. C'est pourquoi le graphite conduit l'électricité.
C'est ce qui le rend utile, car les autres types de carbone ne fonctionnent pas aussi bien. Les propriétés du graphite le distinguent du diamant. Le graphite peut être utilisé dans les appareils électriques rapides. Les électrodes en graphite utilisées dans l'acier et la métallurgie sont fabriquées par Jinsun Carbon, qui produit des électrodes en graphite de haute qualité.
- Structure en couches
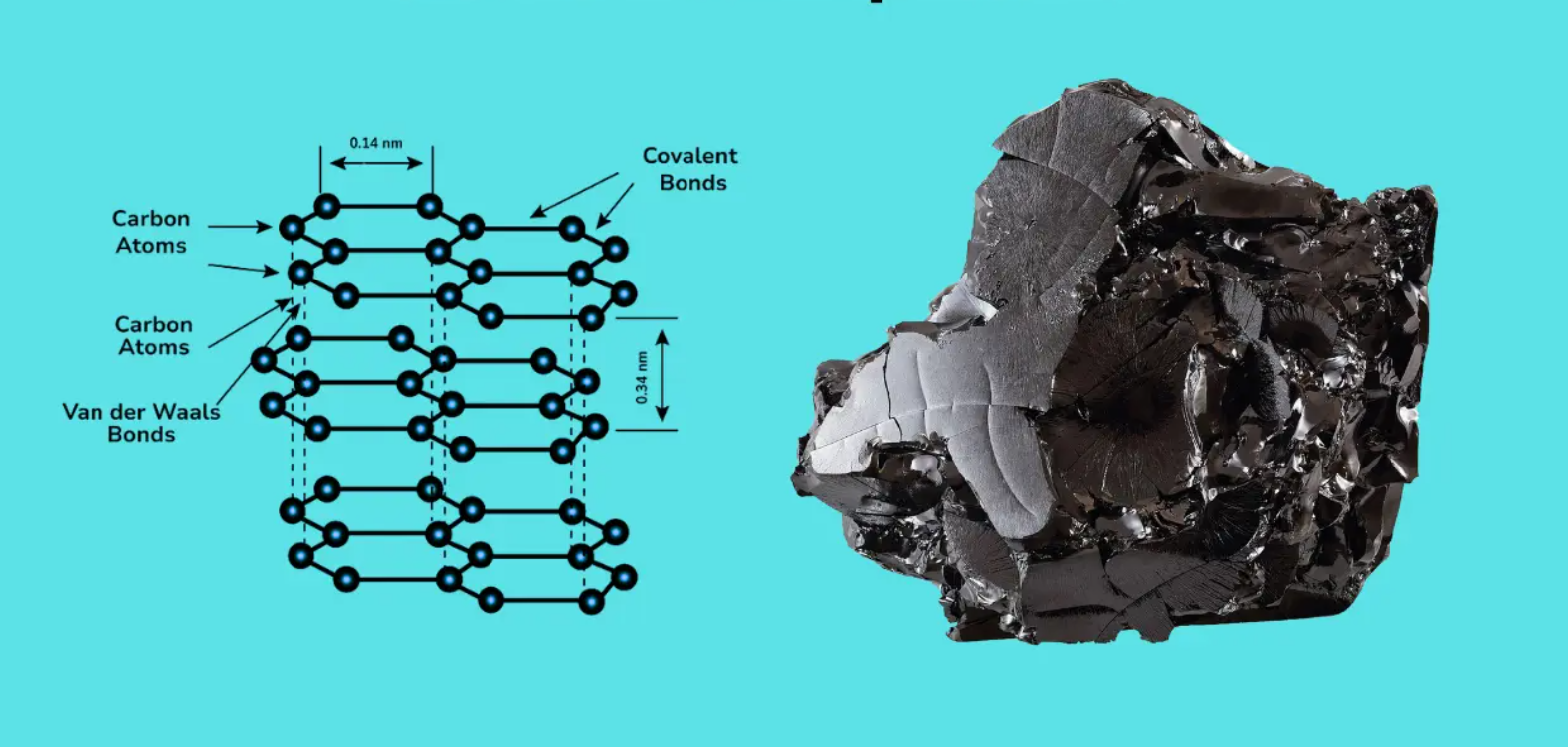
Il y a de nombreuses couches minces de graphite. Les atomes forment un hexagone. Ils glissent facilement. Des forces faibles, les forces de van der Waals, maintiennent les couches. Les couches sont distantes de 3,35 Å.
Cela permet aux électrons de passer d'une couche à l'autre. Chaque couche présente une liaison forte de 1,42 Å entre les atomes de carbone. Les propriétés du graphite le rendent souple et adapté à de nombreuses utilisations. Elles lui permettent de mieux construire l'électricité. Les structures en couches sont utilisées pour les électrodes de Jinsun Carbon afin d'obtenir les meilleures performances.
- Conductivité élevée
Le graphite est un bon conducteur d'électricité. Les électrons π s'y déplacent librement. Ils forment un nuage d'électrons qui fonctionne mieux. La conduction peut atteindre 10³ S/m. On le trouve dans les piles et dans les outils électriques.
En fait, la conduction du graphite est meilleure que celle de la plupart des non-métaux. Les électrons ne sont pas collés à un atome et c'est ainsi que cela fonctionne. On peut même observer ce phénomène dans les outils de tous les jours, tels que les crayons et les piles.
- Conduction anisotrope
Dans le graphite, l'électricité circule différemment selon les directions. Elle est très rapide le long des couches. Ici, la vitesse de l'électricité est de 10⁵ S/m. Elle est plus lente entre les couches où les liaisons sont plus faibles.
C'est ce qui fait du graphite une bonne chose lorsque l'électricité doit circuler dans un sens, car elle ne peut circuler que dans un sens. Cela est possible grâce aux couches. Jinsun Carbon garantit la meilleure qualité de conduction anisotrope pour vos besoins.
- Électrons Π délocalisés
Les électrons π se déplacent sur les couches du graphite. Ils ne restent pas attachés à un seul atome. En fait, le courant se déplace bien à travers le graphite. Les atomes du graphite ont une forme appelée sp². Cela signifie qu'il y a un électron libre. L'électricité peut facilement traverser les couches de la nitrocellulose.
Propriétés thermiques du graphite !
- Conductivité thermique élevée
La raison pour laquelle le graphite est spécial est qu'il déplace la chaleur très rapidement. Il est capable d'envoyer de la chaleur à une vitesse comprise entre 200 et 800 W/m-K. La chaleur voyage loin dans le graphite en raison des couches. Les ingénieurs l'utilisent dans le domaine de l'électronique où les choses peuvent devenir très chaudes. Mais certains types de graphite peuvent atteindre 1 700 W/m-K.
C'est très rapide ! Ces propriétés sont utilisées par des pièces telles que les dissipateurs de chaleur pour refroidir. Les propriétés du graphite le rendent idéal pour évacuer la chaleur des ordinateurs et des lampes.
- Dissipation de la chaleur
Le graphite est fait pour évacuer la chaleur. Dans les environnements chauds, il refroidit très rapidement ; il ne retient pas la chaleur. Il est très utile pour les machines telles que les ordinateurs.
Le graphite peut accepter 700 W/m-K de chaleur. Il permet de diffuser rapidement la chaleur loin des endroits chauds. Ces propriétés du graphite sont importantes pour éviter la surchauffe des machines. Les appareils tels que les unités centrales de traitement et les diodes électroluminescentes (DEL) qui fonctionnent bien en sont la preuve.
- Résistance à la température
Lorsque le graphite est soumis au super compresseur à haute température, il reste solide. Il fond à 3 600 °C et résiste. Il est utilisé dans un environnement comme un four, ou même dans un vaisseau spatial où les choses deviennent trop chaudes. Il est idéal pour les tâches très difficiles, comme dans un four ou une fusée spatiale. Il ne se brise pas non plus lorsqu'il fait froid, ce qui lui permet d'être utilisé dans de nombreux endroits.
- Dilatation thermique
Le graphite ne change pas beaucoup de forme lorsqu'il est chauffé. Il croît à peine, d'environ 1-2 × 10-⁶/°C, et ne se plie pas et ne se fissure pas à haute température. Il est donc idéal pour des objets tels que les ordinateurs, etc., qui doivent s'ajuster très précisément les uns aux autres, même s'ils deviennent très chauds.
- Capacité thermique spécifique
Il ne faut pas beaucoup de chaleur pour maintenir le graphite en place. La capacité thermique spécifique est de 720 J/kg.K. Il doit être pompé avec beaucoup d'énergie pour devenir chaud. On trouve du graphite dans les objets qui stockent la chaleur, comme les batteries.
C'est pourquoi le graphite est utilisé dans les machines énergétiques et métalliques. Il emmagasine la chaleur sans s'échauffer trop rapidement.
| Propriété | Graphite | Cuivre | Aluminium | Acier | Verre | Céramique |
| Conductivité thermique | 150-500 W/m-K | 385 W/m-K | 235 W/m-K | 50 W/m-K | 1,1 W/m-K | 20-30 W/m-K |
| Dissipation de la chaleur | Excellent | Très bon | Bon | Modéré | Pauvre | Juste |
| Résistance à la température | 3,000°C | 1,085°C | 660°C | 1,370°C | 1,200°C | 1,400°C |
| Dilatation thermique | 4-7 ×10-⁶ /°C | 16.5 ×10-⁶ /°C | 23 ×10-⁶ /°C | 11.7 ×10-⁶ /°C | 9 ×10-⁶ /°C | 5-10 ×10-⁶ /°C |
| Capacité thermique spécifique | 0,71 J/g-K | 0,39 J/g-K | 0,90 J/g-K | 0,49 J/g-K | 0,84 J/g-K | 0,76 J/g-K |
| Densité | 2,26 g/cm³ | 8,96 g/cm³ | 2,70 g/cm³ | 7,85 g/cm³ | 2,50 g/cm³ | 2,6-3,0 g/cm³ |
Tableau des propriétés thermiques du graphite !
Propriétés structurelles et atomiques du graphite !
- Treillis hexagonal
Le carbone dans le graphite est vraiment minuscule. Il se présente sous la forme d'un hexagone. Ils sont plats comme des hexagones de papier. Ils sont espacés de 3,35 Å. Des liaisons fortes, appelées liaisons sigma, maintiennent les atomes ensemble. Sous un microscope spécial, on peut voir ce motif hexagonal. Le graphite est un conducteur électrique grâce à ses couches.
Cette forme le rend glissant. Des objets sont fabriqués en graphite, comme les crayons et les machines. Il est solide et flexible, mais jamais solide et rigide. Cette forme hexagonale est importante pour les propriétés du graphite.
- Forces de Der Waals
Le graphite est suffisamment lisse pour être touché. Mais ces couches peuvent glisser en raison de forces faibles. Ces forces sont appelées forces de Van der Waals. C'est une colle molle, qui agit comme une colle entre les couches.
Les couches sont distantes de 3,35 Å. Cela signifie que ces liaisons faibles permettent du graphite comme lubrifiant. Lorsque vous le frottez, les couches se déplacent. C'est pourquoi les propriétés du graphite en font un matériau idéal pour les crayons. Sa souplesse est due à l'importance des forces de van der Waals.
- Structure en couches
Le graphite est une pile de papiers. Les papiers sont en fait des couches d'atomes de carbone. Elles sont disposées en feuilles plates. Ces couches restent séparées les unes des autres parce qu'il existe des forces faibles entre les couches.
Cependant, ils glissent l'un sur l'autre. C'est pourquoi le graphite ne se casse pas facilement. Il est capable de supporter une chaleur allant jusqu'à 3 000 °C. Il est également utile pour les usines, grâce à ces couches résistantes. Les couches ne fondent pas et se plient même. Les couches sont importantes pour la structure et les industries où le graphite est utilisé.
- Feuilles de carbone planaires
Le graphite est composé de fines couches plates. On les appelle des feuilles de carbone. Elles ne sont séparées que de 3,35 Å ; on ne les voit pas, mais elles sont là. C'est ainsi que le graphite devient résistant.
Une fois carbonisé, le graphite devient un bon conducteur de chaleur et d'électricité. Ces couches de carbone confèrent au graphite une certaine souplesse de fabrication, et les usines l'utilisent. Ces couches sont également utilisées pour des éléments tels que les batteries. Le graphite est spécial parce qu'il s'agit de feuilles plates de carbone.
- Hybridation Sp²
Comme le graphène, le graphite est constitué d'atomes de carbone qui se lient dans trois directions. Ces liaisons sont appelées orbitales hybrides Sp². C'est comme des bras qui se tiennent la main. Il y a un électron libre par atome.
Lorsqu'un électron se déplace, il peut contribuer à conduire l'électricité. Les couches sont solides, mais faciles à faire glisser en raison de ces liaisons. C'est pourquoi le graphite est utilisé pour les crayons et les machines. La solidité et le mouvement des couches sont tous deux déterminés par ce système de liaison.
Applications basées sur les propriétés du graphite!
- Creusets
Le graphite est solide. Il peut durer dans des endroits très chauds. Le graphite est utilisé comme matériau de creuset. Il peut supporter une chaleur de 3 000 °C. Dans ces creusets, on peut faire fondre de l'or et de l'argent. Le propriétés du graphite aident à rendre les creusets résistants. Ils ne se cassent pas la première fois que vous les refroidissez.
Toutefois, le graphite est pressé à 1 000 °C. La densité de ces creusets est de 1,7 g/cm³. Mais ils ne se dilatent pas beaucoup, seulement 4,9 x 10-⁶/°C. C'est pourquoi ils durent plus longtemps lorsqu'ils sont utilisés plusieurs fois.
- Matériaux réfractaires
L'acier est fabriqué à partir de matériaux réfractaires. Ces matériaux contiennent du graphite. Par exemple, ils sont utilisés dans des endroits très chauds, jusqu'à 2 500 °C. Ils sont utilisés dans les fours de fabrication de l'acier. Ils sont utilisés dans les fours de fabrication de l'acier.
Les propriétés du graphite protègent les matériaux de la fusion du métal. En effet, le métal devient très chaud. Il y a du graphite 20% à l'intérieur. C'est un ordre de grandeur, un tiers de 300 w/m-K. Les fissures s'arrêtent également sur le graphite. Cela empêche tout de s'ouvrir et d'affaiblir les choses à long terme.
- Électrodes
Fort électrodes sont fabriquées à l'aide de graphite. D'une intensité de 100 000 ampères, ces électrodes peuvent contenir une très grande quantité d'électricité. Elles fonctionnent à 3 000 °C ou plus.
Le graphite possède ce que l'on appelle des couches spéciales, qui permettent à l'électricité de circuler rapidement. Sa densité est de 1,55-1,60 g/cm³ dans chaque électrode. Il protège tout de la chaleur, ainsi que les couches. Dans les grandes machines, on utilise le graphite pour fabriquer de l'acier. Les choses peuvent même devenir très chaudes et le graphite continue à fonctionner.
- Piles
L'énergie est stockée dans les batteries. Le graphite stocke l'énergie en toute sécurité. Il comporte de petites parties appelées anodes, où la batterie conserve l'énergie. Leur capacité énergétique est de 372 mAh/g.
Seul 1% (de graphite) se développe lors de la charge. Le problème du graphite est qu'il ne fond qu'à 3 550 °C, mais qu'il est très résistant. Seuls ces minuscules morceaux de graphite, d'une taille de 10 à 25 microns, empêchent l'énergie de circuler correctement dans la batterie. Ils empêchent l'énergie de circuler librement dans la batterie.
- Joints mécaniques
Les garnitures mécaniques arrêtent les fuites. Les joints en graphite sont résistants et ne s'usent pas. Même à une température de 2 500 °C, ils peuvent faire leur travail. Il est dur, 2,2 g/cm³, un joint contre les produits chimiques. Le joint est glissant, en graphite, et ne nécessite donc pas d'huile. Cela permet à la machine de fonctionner très longtemps sans tomber.
Conclusion
Le graphite est utile. Même sous l'effet de la chaleur, il reste solide. Les propriétés du graphite lui permettent de fonctionner dans les machines. Il entre dans la composition des piles et d'autres objets. Découvrez les informations sur le graphite ! En fait, vous pouvez en savoir plus à l'adresse suivante JINSUNCARBON dès maintenant.