Вступ
Чим особливий графіт? Він міцний і слизький. Легко проводить електрику. І стійкий до високотемпературної корозії. Більше про властивості графіту ви дізнаєтесь тут. Ми ж розглянемо ближче, що робить графіт таким ідеальним матеріалом для використання в машинах. Якщо ви хочете дізнатися, як графіт допомагає інженерам, читайте далі.
Зміст
Перемикач
Розуміння фізичних властивостей графіту!
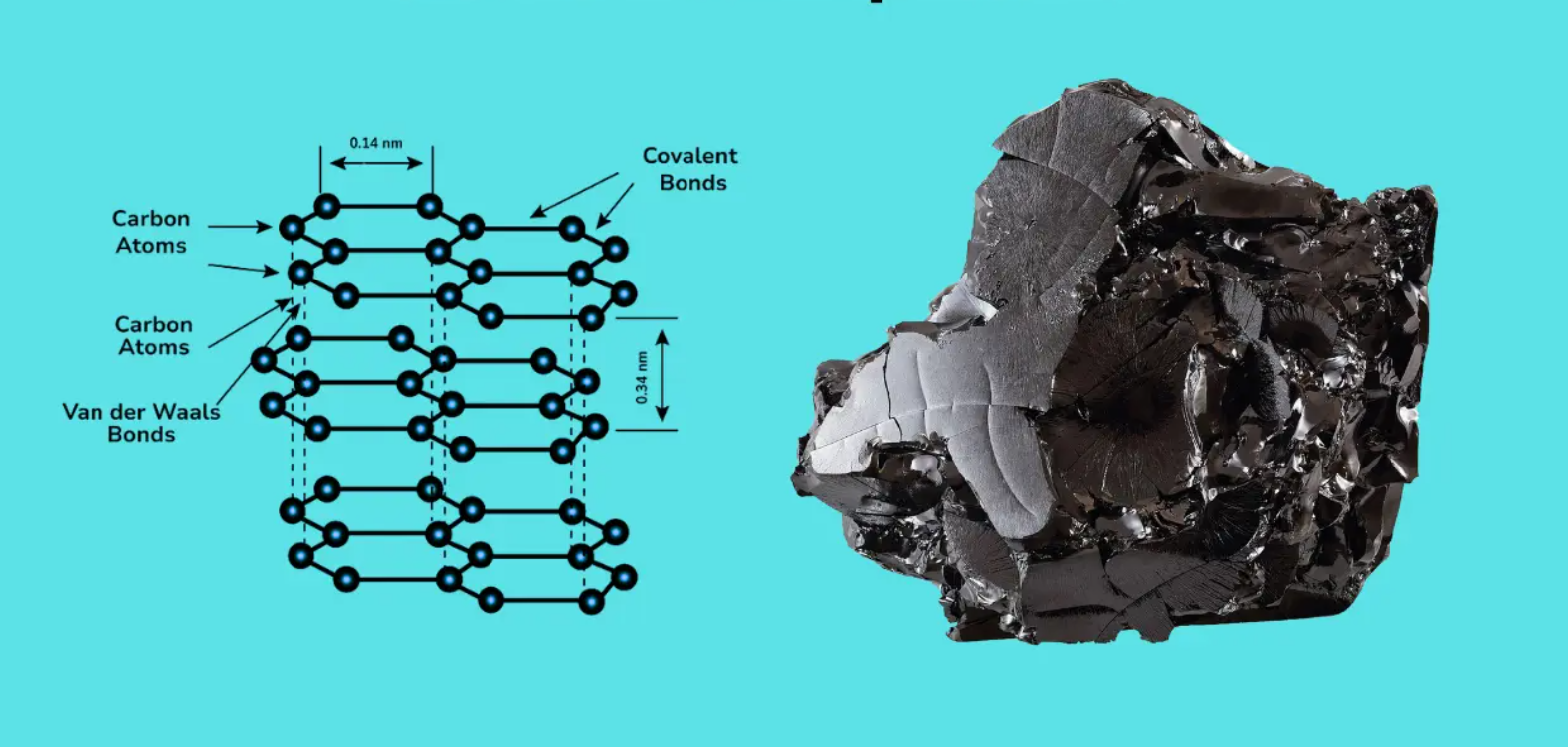
Графіт складається з вуглецю. Шари м'які і легко рухаються. Кожен шар плаский. До властивостей графіту належить його легка вага, близько 2,26 г/см³. Ви можете простягнути руку і доторкнутися до нього, і він слизький. Саме так його використовують для передачі електрики. Графіт також використовується в батарейках та олівцях.
Мало того, що він плавиться лише при надвисокій температурі 3550°C, він ще й дуже міцний. Саме ця особливість робить його гарним матеріалом для машин. Графіт відрізняється від алмазу, але є досить корисним. Вуглець також буває різних відтінків; вони обидва є, але працюють по-різному.
Хімічні властивості графіту!
- Висока інертність
Графіт нелегко змінити. Більшість хімічних речовин не змінюються. Електрони, що сидять на атомах вуглецю в графіті, дуже сильні. Графіт безпечний, тому що вони розташовані шарами. Він не піддається корозії в кислотах і лугах. З іншого боку, ви можете використовувати графіт на заводах, і він працює при 3600°C!
Ось чому він добре підходить для великих машин. Він працює при дуже високих температурах, йому можна довіряти. В ядерних реакторах графіт також міцний. Ви побачите, що він служить довше, ніж будь-який інший матеріал. Ось як Jinsun Carbon виготовляє графітові електроди, які все ще можуть працювати на заводах в умовах високої температури протягом тривалого часу.
- Стійкість до окислення
Графіт міцний. Він не ламається при нагріванні. Нижче 600°C він не реагує з киснем. Це означає, що він безпечний для заводів. Він використовується в печах (якщо ви використовували його при створенні електроди).
До властивостей графіту належить його міцність при нагріванні. Це тому, що графіт захищає себе від руйнування і служить довше. На металургійних заводах, де дуже висока температура, ви хочете використовувати його. Все залишається безпечним і міцним. Jinsun Carbon надає графітові електроди для металургійних заводів, які безпечно витримують екстремальну температуру.
- Кислотостійкість
Графіт безпечний у кислотах. Він не вступає в реакцію, коли ви додаєте сильну кислоту, наприклад, сірчану. Він міститься в акумуляторах або на хімічних заводах. Цей матеріал добре працює у важких умовах.
Графіт довговічний, бо його атоми дуже міцно пов'язані між собою. Властивості графіту означають, що він не змінюється навіть у кислоті. Він підтримує машини в робочому стані. У багатьох галузях промисловості, де інші матеріали можна зруйнувати лише сильною кислотою, використовують саме його.
- Стійкість до лугів
У лузі графіт залишається міцним. Він не реагує на сильні хімічні речовини, такі як гідроксид натрію. Його важко зруйнувати. Вам потрібно, щоб речі на вашій фабриці служили довго.
Графіт протистоїть хімічним речовинам, які можуть пошкодити інші речі. Графіт безпечний, тому що атоми вуглецю роблять його таким. Він має високу стійкість до лугів; його можна використовувати там, де інші матеріали не протримаються довго. Він працює, коли щось йде не так, але продовжує працювати, коли все йде добре.
- Термічна стабільність
Графіт стійкий до нагрівання. Він може витримувати температуру до 3600°C. Він не плавиться. Але вуглецеві шари залишаються міцними та безпечними. Його можна використовувати в дуже гарячих машинах, таких як теплові екрани та деталі ракет.
Тепло також у графіті йде добре. Ось чому він в електроніці. Він охолоджує машини. Його термостійкість - одна з найкращих властивостей графіту. Він знаходиться в багатьох місцях з високою температурою, тому речі працюють краще. Ці екстремальні умови? Без проблем, Jinsun Carbon's графітові вироби перевершують всі інші.
| Власність | Графіт | Інертність | Стійкість до окислення | Кислотостійкість | Стійкість до лугів | Термічна стабільність |
| Рівень інертності | Високий | 9/10 | Середній | Високий | Високий | Чудово. |
| Температура окислення. | > 600°C | Н/Д | Так. | Обмежений | Так. | До 3000°C |
| Кислотна реакція | Стійкий | Ніякої реакції. | Мінор. | Стабільно. | Ніякої реакції. | Стабільно. |
| Лужна реакція | Стійкий | Ніякої реакції. | Так. | Так. | Стабільно. | Стабільно. |
| Теплопровідність | 100-400 Вт/мК | Немає ефекту | Деяка деградація | Немає ефекту | Мінімальний вплив | Залишається високим |
| Структурна цілісність | Міцні зв'язки | Підтримується | Без суттєвих змін | Ніяких пошкоджень. | Підтримується | Залишається неушкодженим |
Таблиця хімічних властивостей графіту!
Механічні властивості та міцність графіту!
- Низька міцність на розрив
Графіт, що розривається на частини, не є міцним. Це те, що може зламатися при зусиллі 20-25 МПа. Коли ви тягнете за графіт, він ламається, бо не витримує такої сили. При розтягуванні його вуглецеві шари ковзають. Понад 500 МПа міцність на розрив - це вже міцніші речі: сталь, наприклад.
Коли ви думаєте про властивості графіту, пам'ятайте, що він погано витримує розтягнення. Він легко ламається, якщо тягнути його занадто сильно. Міцність на розрив означає, наскільки сильно можна розтягнути щось, перш ніж воно зламається.
- Висока міцність на стиск
Якщо натиснути на графіт, він стає дуже міцним. Ця штука може тиснути на вас до 150 МПа. Він залишається міцним, якщо на нього натиснути. Навіть під великою силою шари графіту важко розчавити. Він міцний, тому що гексагональні атоми допомагають йому залишатися міцним, коли його стискають.
Коли ви думаєте про властивості графіту, ви бачите, що він добре справляється з тиском. Його міцність і міцність на стиск роблять його хорошим кандидатом для використання в ущільненнях, які повинні залишатися герметичними.
- Анізотропна поведінка
Для графіту має значення, як ви натискаєте на нього. Він легко розривається в одному напрямку. В іншому він міцний проти стискання. За словами інженерів, це анізотропія.
Якщо їх розтягнути, то шари вуглецю розсунуться, але витримають тиск. Ці властивості графіту роблять його особливим матеріалом для таких речей, як електроди. Він навіть проводить електрику краще в один бік, ніж в інший.
- Модуль пружності
Графіт гнеться під тиском, але не дуже сильно. Його модуль пружності становить 10-15 ГПа. Модуль пружності говорить вам про жорсткість. Графіт трохи прогинається, коли ви на нього тиснете, але він пружинить назад.
Наприклад, сталь набагато жорсткіша при 200 ГПа. Вона м'якша, з огляду на те, що вона є, але, тим не менш, має хорошу міцність. Її еластичність означає, що вона може згинатися і знову розмокати.
- В'язкість при руйнуванні
Якщо натиснути на графіт занадто сильно, він легко тріскається. Його в'язкість при руйнуванні становить від 0,5 до 1,5 МПа-м¹/². Якщо ви не будете обережними, натиснете на нього, і він швидко трісне. Як тільки тріщина починається, вона поширюється.
Графіт погано сприймає тріщини, тому інженери повинні поводитися з ним обережно. Він добре тримається під тиском, але ламається, якщо його потягнути або вдарити занадто сильно. З точки зору в'язкості руйнування, ви знаєте, як сильно матеріал може витримати удар, перш ніж він втратить свою форму.
| Власність | Міцність на розрив | Міцність на стиск | Анізотропна поведінка | Модуль пружності | В'язкість при руйнуванні |
| Одиниця | МПа | МПа | Змінюється (площини XY) | ГПа | МПа-м^0.5 |
| Значення | Низький (≈ 20-30 МПа) | Висока (≈ 100-200 МПа) | Так. | Помірний (≈ 8-12 ГПа) | Низький (≈ 1-2 МПа-м^0,5) |
| Спрямованість | Ізотропність (низька) | Варіюється | Високий | Варіюється | Варіюється |
| Вплив застосування | Крихкі структури | Структурна підтримка | Термостійкість | Межі деформації | Стійкість до руйнування |
| Температурні ефекти | Зниження | Збільшення | Так. | Зменшує | Зменшує |
| Використання | Мастила, ущільнювачі | Вогнетривкі матеріали | Теплові екрани | Датчики | Стресові програми |
Таблиця механічних властивостей та міцності графіту!
Електропровідність графіту!
- Вільні електрони
Графіт має вільні електрони. Три з чотирьох атомів вуглецю мають три електрони, які беруть участь у зв'язках. Один електрон рухається вільно. Це змушує електрику рухатися. В одному см³ графіту міститься близько 6 x 10¹⁸ вільних електронів. Вони ходять туди-сюди між шарами. Ось чому він проводить електричний струм.
Це робить його корисним, адже інші види вуглецю працюють не так добре. Властивості графіту відрізняють його від алмазів. Графіт можна використовувати в електричних пристроях, що швидко рухаються. Графітові електроди, що використовуються в металургії, виготовляються компанією Jinsun Carbon, яка виробляє високоякісні графітові електроди.
- Шарувата структура
Існує багато тонких шарів графіту. Атоми утворюють шестикутник. Вони легко ковзають. Слабкі сили, сили Ван-дер-Ваальса, утримують шари. Шари знаходяться на відстані 3,35 Å один від одного.
Це дозволяє електронам проникати між шарами. Кожен шар демонструє міцний зв'язок у 1,42 Å між атомами вуглецю. Властивості графіту роблять його м'яким і придатним для багатьох застосувань. Це допомагає йому краще конструювати електрику. Шаруваті структури використовуються для електродів Jinsun Carbon для досягнення найвищої продуктивності.
- Висока провідність
Графіт - хороший провідник електрики. У ньому π-електрони вільно рухаються. Вони утворюють електронну хмару, яка працює краще. Провідність до 10³ См/м. Його можна знайти в акумуляторах і в електричних інструментах.
Насправді, провідність графіту краща, ніж у більшості неметалів. Електрони не затримуються на жодному атомі, і саме так він працює. Ви навіть можете побачити цей повсякденний інструмент, такий як олівці та батарейки.
- Анізотропна провідність
У графіті електрика тече по-різному в різних напрямках. Вона дуже швидка вздовж шарів. Тут ми маємо швидкість електрики 10⁵ См/м. Між шарами, де зв'язки слабші, вона повільніша.
І саме це робить графіт гарною річчю, коли вам потрібно, щоб електрика була односторонньою, тому що вона може текти лише в одному напрямку. Це можливо завдяки шарам. Найкращу якість анізотропної провідності для ваших потреб гарантує Jinsun Carbon.
- Делокалізовані Π-електрони
У графіті π-електрони рухаються між шарами. Вони не прилипають до одного атома. Насправді, струм добре проходить крізь графіт. Атоми в графіті мають таку форму, яка називається sp². Це означає, що у них є один вільний електрон. Електрика може легко проходити крізь шари нітроцелюлози.
Теплові властивості графіту!
- Висока теплопровідність
Особливість графіту полягає в тому, що він дуже швидко переносить тепло. Він здатен передавати тепло зі швидкістю від 200 до 800 Вт/м-К. Тепло в графіті поширюється на велику відстань завдяки шарам. Інженери використовують його в електроніці, де речі можуть дуже сильно нагріватися. Але бачите, деякі види графіту можуть досягати 1 700 Вт/м-К.
Це дуже швидко! Ці властивості використовуються в таких деталях, як радіатори для охолодження. Властивості графіту роблять його чудовим матеріалом для відведення тепла від комп'ютерів та ламп.
- Відведення тепла
Позбавлятися тепла - ось для чого створений графіт. У гарячому середовищі він дуже швидко охолоджується, не утримує тепло. Це дуже добре для машин, таких як комп'ютери.
Графіт може приймати 700 Вт/м-К тепла. Це дозволяє швидко поширювати тепло далеко від гарячих місць. Як бачите, такі властивості графіту є важливими для запобігання перегріву машин. Пристрої, такі як процесори та світлодіоди, які працюють дуже довго, можуть це довести.
- Стійкість до температури
Коли ви поміщаєте графіт під розпечений суперкомпресор, він залишається міцним. Він плавиться при температурі 3600°C і витримує це. Його використовують у таких середовищах, як піч, або навіть на космічних кораблях, де дуже спекотно. Він чудово підходить для дуже важких робіт, наприклад, у печі або космічній ракеті. Він не ламається, коли стає холодно, тож може використовуватися в багатьох місцях.
- Теплове розширення
Графіт майже не змінює форму при нагріванні. Він майже не росте, лише приблизно на 1-2 × 10-⁶/°C, і не гнеться і не тріскається при високих температурах. Тому він чудово підходить для таких речей, як комп'ютери тощо, які мають бути дуже точно підігнані одна до одної, навіть якщо вони гарячі зверху.
- Питома теплоємність
Щоб утримувати графіт, не потрібно багато тепла. Питома теплоємність становить 720 Дж/кг.К. Щоб нагріти графіт, його потрібно накачати великою кількістю енергії. Графіт можна знайти в речах, які зберігають тепло, наприклад, в батареях.
Саме тому графіт використовується в енергетиці та металургії. Він зберігає тепло, не нагріваючись так швидко.
| Власність | Графіт | Мідь | Алюміній | Сталь | Скло | Кераміка |
| Теплопровідність | 150-500 Вт/м-К | 385 Вт/м-К | 235 Вт/м-К | 50 Вт/м-К | 1,1 Вт/м-К | 20-30 Вт/м-К |
| Відведення тепла | Чудово. | Дуже добре. | Добре. | Помірний | Бідолаха. | Справедливо |
| Стійкість до температури | 3,000°C | 1,085°C | 660°C | 1,370°C | 1,200°C | 1,400°C |
| Теплове розширення | 4-7 ×10-⁶ /°C | 16.5 ×10-⁶ /°C | 23 ×10-⁶ /°C | 11.7 ×10-⁶ /°C | 9 ×10-⁶ /°C | 5-10 ×10-⁶ /°C |
| Питома теплоємність | 0,71 Дж/г-К | 0,39 Дж/г-К | 0,90 Дж/г-К | 0,49 Дж/г-К | 0,84 Дж/г-К | 0,76 Дж/г-К |
| Щільність | 2,26 г/см3 | 8,96 г/см3 | 2,70 г/см3 | 7,85 г/см3 | 2,50 г/см3 | 2,6-3,0 г/см³. |
Таблиця термічних властивостей графіту!
Структурні та атомні властивості графіту!
- Гексагональна решітка
Вуглець у графіті дуже крихітний. Він сидить у формі шестикутника. Це плоскі, як паперові шестикутники. Відстань між ними 3,35 Å. Сильні зв'язки називаються сигма-зв'язками, які утримують атоми разом. Під спеціальним мікроскопом можна побачити цей шестикутний візерунок. Графіт є електричним провідником через свої шари.
Така форма робить його слизьким. З графіту роблять речі, наприклад, олівці та машини. Він міцний і гнучкий, але ніколи не буває міцним і жорстким. Ця шестикутна форма важлива для властивостей графіту.
- Сили ан дер Ваальса
Графіт досить гладкий на дотик. Але ці шари можуть ковзати через слабкі сили. Ці сили називаються силами Ван-дер-Ваальса. Це м'який клей, і він діє як клей між шарами.
Шари знаходяться на відстані 3,35 Å один від одного. Це означає, що ці слабкі зв'язки дозволяють графіт, що виконує роль мастила. Коли ви натираєте його, шари рухаються. Саме тому властивості графіту роблять його ідеальним матеріалом для олівців. Його м'якість пояснюється важливістю сил Ван-дер-Ваальса.
- Шарувата структура
Графіт - це стос паперу. Папірці - це, власне, шари атомів вуглецю. Вони розташовуються у вигляді пласких аркушів. Ці шари тримаються окремо, тому що між ними існують слабкі сили.
Однак вони ковзають одна повз одну. Тому графіт не так легко ламається. Він здатен витримувати температуру до 3 000°C. Завдяки цим міцним шарам він також корисний для заводів. Шари не плавляться і навіть згинаються. Шари є важливими для конструкцій та галузей промисловості, де використовується графіт.
- Плоский вуглецевий лист
Графіт складається з тонких пласких шарів. Вони називаються вуглецевими листами. Вони знаходяться на відстані лише 3,35 Å один від одного; їх не видно, але вони є. Так графіт стає міцним.
Після карбонізації графіт стає гарним провідником тепла та електрики. Ці шари вуглецю надають гнучкості у виробництві графіту, і заводи використовують його. Ці шари також застосовуються для таких речей, як батареї. Графіт особливий тим, що складається з пласких листів вуглецю.
- Гібридизація Sp²
Як і графен, графіт складається з атомів вуглецю, які з'єднуються в трьох напрямках. Ці зв'язки називаються гібридними орбіталями Sp². Це як руки, що тримаються за руки. На кожен атом припадає один вільний електрон.
Коли електрон рухається навколо, він може допомагати проводити електрику. Шари міцні, але легко ковзають через ці зв'язки. Саме тому графіт використовують для олівців і машин. Міцність і рух шарів визначаються цією системою зв'язків.
Застосування на основі властивостей графіту!
- Тиглі
Графіт міцний. Він може витримувати дуже високі температури. Графіт використовується як матеріал для тиглів. Він витримує температуру до 3 000°C. У таких тиглях можна плавити золото і срібло. У тиглях з графіту плавлять золото і срібло. властивості графіту допомагають зробити тиглі міцними. Вони не розбиваються при першому ж охолодженні.
Однак графіт пресується у форму при температурі 1,000°C. Щільність цих тиглів становить 1,7 г/см³. Але вони не сильно розширюються, лише на 4,9 x 10-⁶/°C. Тому вони служать довше при багаторазовому використанні.
- Вогнетривкі матеріали
Сталь виготовляється з вогнетривких матеріалів. До складу цих матеріалів входить графіт. Наприклад, вони працюють у дуже гарячих місцях до 2 500°C. У сталеплавильних печах.
Властивості графіту захищають матеріали від розплавлення металу. Це тому, що метал дуже сильно нагрівається. Всередині знаходиться графіт 20%. Це на порядок більше, третина від 300 Вт/м-К. Тріщини також зупиняються на графіті. Це запобігає тому, що все розлітається і послаблює речі на довгий час.
- Електроди
Сильний електроди виготовляються за допомогою графіту. Ці електроди можуть утримувати дуже велику силу струму, наприклад, 100 000 ампер. Вони працюють при температурі 3,000°C і вище.
Графіт має так звані спеціальні шари, які дозволяють електриці рухатися швидко. Його щільність становить 1,55-1,60 г/см³ в кожному електроді. Він захищає все від нагрівання, і шари також. У великих машинах графіт використовується для виробництва сталі. Речі можуть навіть нагріватися до надвисокої температури, а він все одно продовжує працювати.
- Батареї
Енергія зберігається в батареях. Графіт надійно зберігає енергію. Він має маленькі частини, які називаються анодами, де батарея зберігає енергію. Вони мають ємність 372 мАг/г.
Тільки 1% (з графіту) зростає під час заряджання. Проблема графіту в тому, що він плавиться лише при 3550°C, але дуже міцний. Лише ці крихітні шматочки графіту, розміром 10-25 мікрон. Вони заважають енергії безперешкодно протікати в акумуляторі.
- Механічні ущільнення
Механічні ущільнення зупиняють витоки. Графітові ущільнення міцні та не зношуються, тому ви можете їх використовувати. Навіть при температурі 2 500°C вони можуть виконувати свою роботу. Це тверде, 2,2 г/см³, ущільнення, стійке до хімічних речовин. Ущільнення слизьке, виготовлене з графіту, тому воно не потребує мастила. Це дозволяє машині працювати дуже довго, не допускаючи її падіння.
Висновок
Графіт корисний. Навіть у спеку він залишається міцним. Властивості графіту допомагають йому працювати в машинах. Він є в акумуляторах та інших речах. Дізнайтеся більше про графіт! Дізнайтеся більше на ДЖИНСУНКАРБОН прямо зараз.