O ponto de fusão do grafite é importante para a açoO grafite derrete e é usado nos setores de baterias e semicondutores. O grafite derrete? Essa pergunta é frequentemente usada em viagens para explorar o grafite. As características de resistência a altas temperaturas conferidas pelo ponto de fusão do grafite estabelecem uma base sólida para avanços tecnológicos e inovações de processos em muitos campos.
Índice
AlternarO valor específico do ponto de fusão do grafite
O ponto de fusão do grafite é um valor alto na escala de temperatura Fahrenheit. Normalmente, o ponto de fusão do grafite é de aproximadamente 6332°F.
O valor aceito do ponto de fusão do grafite
Na pesquisa científica, o ponto de fusão aceito do grafite é de aproximadamente 3652-3697 °C à pressão atmosférica padrão (diferentes condições experimentais e métodos de medição podem levar a pequenas diferenças nos valores). Essa faixa de temperatura mostra que o grafite pode suportar características de calor extremamente altas. E também mostra que ele é um material resistente a altas temperaturas.
Compare os pontos de fusão de outros alótropos de carbono
Em comparação com o grafite, o ponto de fusão do diamante (diamante) também é muito alto. O ponto de fusão do diamante é de aproximadamente 4000 °C. Isso ocorre porque a estrutura cristalina do diamante e do grafite é diferente. O diamante é um cristal atômico típico, cada átomo de carbono e os quatro átomos de carbono adjacentes têm ligação covalente. O grafite é um cristal misto, que tem ligações covalentes e forças intermoleculares, mas o calor geral de fusão ainda é muito alto.
Fatores que afetam o ponto de fusão do grafite
A estrutura cristalina é dominante
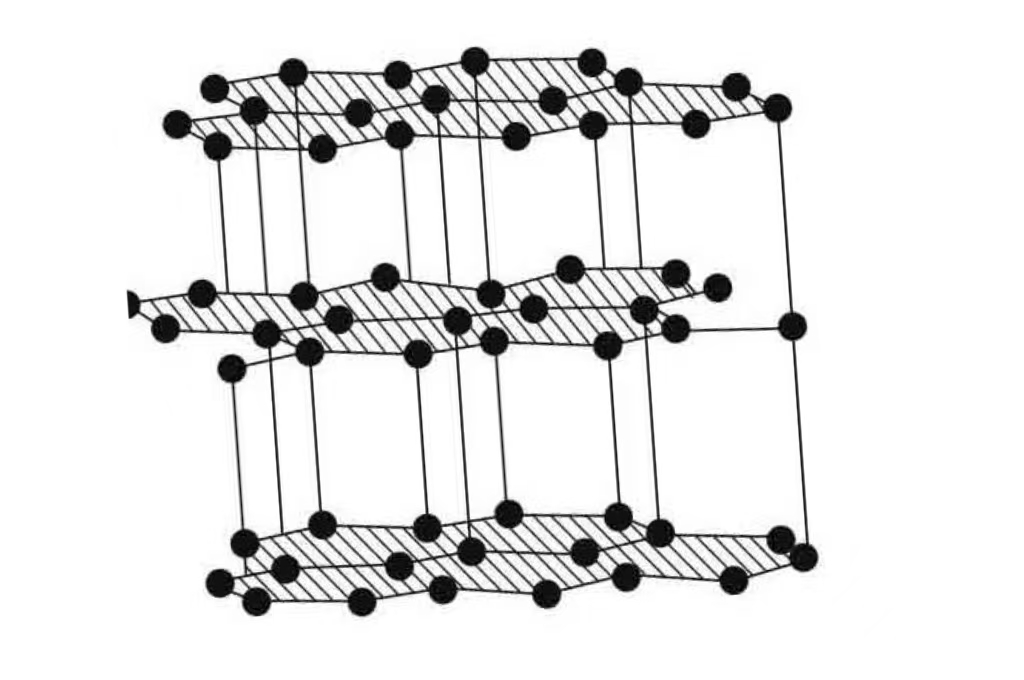
O alto ponto de fusão do grafite está relacionado principalmente à sua estrutura. O cristal de grafite é uma estrutura de rede planar hexagonal composta de ligações covalentes entre átomos de carbono, com forças fracas de van der Waals entre as camadas. Durante o aquecimento, essas ligações covalentes precisam ser superadas para que o grafite derreta. E como essas ligações covalentes são fortes, elas precisam de temperaturas muito altas para serem alcançadas. Ao mesmo tempo, a pressão externa e outros fatores também terão um certo impacto sobre o ponto de fusão, mas, na pressão atmosférica padrão, esse efeito é relativamente pequeno.
Interferência de elementos de impureza
Se o grafite contiver elementos de impureza, como boro, nitrogênio, etc., o grafite pode ser usado para a fabricação de produtos de limpeza, ele alterará a integridade da rede e a energia de ligação química do cristal de grafite e, em seguida, afetará o ponto de fusão. As impurezas podem destruir a homogeneidade da ligação covalente até certo ponto, tornando o ponto de fusão mais baixo ou mais alto, dependendo do tipo e do conteúdo das impurezas.
Correlação com o ambiente externo
Embora o efeito da pressão seja relativamente pequeno na pressão atmosférica padrão. Mas o ambiente de pressão extremamente alta comprime a estrutura cristalina do grafite e aumenta a interação interatômica. Dessa forma, aumenta o ponto de fusão. Em uma atmosfera específica, se a atmosfera reagir com o grafite, a estrutura da superfície e a composição do grafite poderão ser alteradas. Isso afetará sua estabilidade térmica e seu ponto de fusão.
A importância do ponto de fusão do grafite em aplicações práticas
O cadinho de grafite
O cadinho de grafite aproveita o alto ponto de fusão do grafite. Na indústria metalúrgica, é necessário derreter vários metais, como cobre, ferro, etc., o cadinho de grafite pode suportar a alta temperatura quando o metal é derretido e não reage com o metal para garantir a pureza do metal. Em experimentos químicos de alta temperatura, o alto ponto de fusão do grafite também o torna um material ideal para vasos de reação. No setor de eletrônicos, o grafite também é usado para dissipação de calor de componentes eletrônicos em ambientes de alta temperatura. Devido à sua resistência a altas temperaturas, ele pode resistir aos danos causados por altas temperaturas em sua própria estrutura e desempenho até certo ponto. Conduz eficazmente o calor para fora e garante a operação normal dos componentes eletrônicos.
No campo aeroespacial
Na engenharia aeroespacial, os bicos de foguetes enfrentam temperaturas extremamente altas e fluxo de ar de alta velocidade. Com seu alto ponto de fusão, o grafite pode suportar a alta temperatura de milhares de graus Celsius no bocal e garantir a estabilidade da estrutura. Ao mesmo tempo, ele evita danos ao bocal devido ao derretimento do material e garante a propulsão suave do foguete.
Blocos de grafite em fornos metalúrgicos e de resistência
Blocos de grafite em fornos metalúrgicos e fornos de resistência Aproveitar ao máximo as características de alto ponto de fusão do grafite. Na fundição de cobre, ferro e outros metais, o bloco de grafite pode suportar altas temperaturas por um longo tempo, mantendo o ambiente de alta temperatura no forno. Além disso, ele não reage com o metal para garantir a qualidade e a pureza da fundição do metal.
Bloco de carbono do cátodo de grafite na eletrólise do alumínio
O grafite cátodo O bloco de carbono desempenha um papel fundamental no processo do alumínio eletrólise. Seu alto ponto de fusão o torna estável em ambiente eletrolítico de alta temperatura, proporcionando uma interface de eletrodo adequada para a redução de íons de alumínio. Além disso, ele resiste à erosão do eletrólito e ao impacto de alta temperatura para garantir uma operação eficiente e estável da produção eletrolítica de alumínio.
Conclusão
O grafite tem um alto ponto de fusão. Suas características de ponto de fusão estão intimamente relacionadas à sua estrutura interna e também são únicas em comparação com os pontos de fusão de outros alótropos de carbono, como o diamante. A pesquisa e a utilização do ponto de fusão do grafite nos ajudarão a aproveitar melhor as vantagens do grafite. E, em seguida, expandir sua aplicação em mais campos relacionados ao ambiente de alta temperatura.