El grafito, como alótropo del carbono, aparece con frecuencia en la vida cotidiana y en la producción industrial. ¿Qué es el grafito? ¿Es un metal, un mineral o un elemento? ¿De qué está hecho? Este artículo explora en profundidad la naturaleza del grafito, desvela sus secretos. Y mostrará plenamente el encanto único y el importante valor del grafito en el campo de la ciencia y la aplicación.
Índice
ToggleDefinición de grafito
El grafito es una forma cristalina del carbono, que suele mostrar un estado sólido gris-negro y opaco, con un brillo metálico único. Pero a veces tiene otra forma que es la forma amorfa, que consiste en disposicionesirreeulares de átomos de grafito. Su textura es relativamente blanda, esta blandura le permite dejar marcas claras en el papel. Esta propiedad también hace que el grafito se convierta en el principal componente de la mina de los lápices. En esencia química, el grafito pertenece al alótropo del carbono, que se compone de átomos de carbono como el diamante, el fullereno y otras sustancias. Sin embargo, la disposición de los átomos de carbono en estas sustancias es muy diferente, lo que da lugar a enormes diferencias en sus propiedades físicas y químicas.
¿El grafito es un metal o un mineral?
El grafito no es un metal, sino un mineral. Los metales suelen tener buena conductividad eléctrica, conductividad térmica y ductilidad y otras características típicas. Aunque el grafito tiene cierto grado de conductividad eléctrica y térmica, no tiene la ductilidad característica de los metales. El grafito es un producto natural de procesos geológicos complejos y responde a la definición de mineral. Existe en rocas y depósitos específicos de la naturaleza. Y es una forma importante de carbono en el largo ciclo y proceso de evolución del círculo terrestre, testigo del movimiento y cambio de materiales en el interior de la Tierra.
¿Es el grafito un elemento?
El grafito no es un elemento, sino una sustancia compuesta de carbono. Un elemento es un grupo de átomos que tienen el mismo número de cargas nucleares (protones). El grafito es una entidad material formada por un gran número de átomos de carbono unidos por enlaces químicos específicos. Esta sustancia pura compuesta por los mismos elementos se denomina elemental. El grafito es una forma especial de existencia elemental del carbono. Con una estructura cristalina y unas propiedades físicas y químicas únicas, muestra las ricas y diversas propiedades del carbono.
¿El grafito es carbono?
El grafito es un alótropo del carbono, compuesto en su totalidad por el elemento carbono. En el interior del grafito, los átomos de carbono están dispuestos y combinados de una manera muy especial para formar una estructura cristalina única. Esta estructura confiere al grafito muchas propiedades especiales. Por eso, su aspecto y sus propiedades físicas y químicas lo diferencian significativamente de otros alótropos del carbono, como el diamante (conocido por su dureza y transparencia) o el fullereno (con una estructura esférica o tubular única). En muchos campos desempeñan papeles diferentes.
¿De dónde procede el grafito?
Fuentes naturales
Las fuentes naturales de grafito en la naturaleza son más extensas. Podemos encontrar parte del grafito en rocas metamórficas. Por ejemplo, en el proceso de metamorfismo regional, los sedimentos originales que contienen carbono (como los filones de carbón) se transforman gradualmente en grafito mediante una compleja cristalización metamórfica en condiciones extremas de alta temperatura y presión. Además, parte del grafito procede de rocas magmáticas. Y cuando el magma invade la corteza, el carbono transportado en el magma cristaliza en un entorno geológico y unas condiciones físicas y químicas específicas. Así se forma el grafito. Los yacimientos naturales de grafito están distribuidos en muchos países y regiones de todo el mundo, entre los que China, Brasil, India y otros países cuentan con recursos naturales de grafito relativamente ricos. Éstos proporcionan una importante base material para el desarrollo de las industrias mundiales relacionadas con el grafito.
Grafito artificial
Con el rápido desarrollo de la tecnología industrial moderna, la producción de grafito artificial se ha convertido gradualmente en una parte importante del suministro de grafito. El grafito artificial suele producirse mediante el tratamiento térmico a alta temperatura de materias primas específicas que contienen carbono (como el coque de petróleo, el coque de asfalto, etc.). Convierte amorfo carbono en grafito. A altas temperaturas, los elementos no carbonosos de estas materias primas que contienen carbono se volatilizarán gradualmente. Y los átomos de carbono se reorganizarán y cristalizarán, formando finalmente grafito artificial con una estructura similar a la del grafito natural. La producción de grafito artificial tiene un alto grado de controlabilidad. Y puede controlar con precisión su pureza, estructura cristalina y propiedades físicas y químicas según las diferentes necesidades de aplicación industrial. De modo que se utiliza ampliamente en muchos campos como el acero, las baterías, los refractarios, etc. Esto proporciona un fuerte apoyo para el desarrollo de la industria moderna.
Tipos de grafito
En resumen, el grafito puede dividirse principalmente en dos tipos: grafito natural y grafito sintético.
Grafito natural
El grafito natural también incluye el grafito en escamas, el grafito cristalino y el grafito criptocristalino.
El grafito en escamas se caracteriza por su forma de escamas grandes y finas con una amplia gama de diámetros, que van desde unas décimas de milímetro hasta varios milímetros. Estas escamas presentan una buena conductividad eléctrica y térmica en la dirección del plano, mientras que su estructura en capas le confiere excelentes propiedades de lubricación.
Los cristales de grafito cristalino están bien desarrollados y muestran una evidente forma de cristal laminar hexagonal. Y sus escamas son relativamente grandes, y el diámetro de las láminas suele ser superior a 0,1-0,2 mm. Este tipo de grafito no abunda en la naturaleza. Pero debido a su excelente estructura cristalina y a sus propiedades únicas, desempeña un papel importante en muchos campos industriales de alta gama.
El cristal de grafito afanítico es muy pequeño y existe en forma de agregados microcristalinos. Y la forma específica del cristal apenas puede distinguirse a simple vista. Su contenido en carbono fijo es elevado, aproximadamente del orden de 60%-80%. En aplicaciones industriales, podemos utilizar el grafito criptocristalino como material de fundición y refractario.
Grafito sintético
El grafito sintético es de tres tipos, cada uno con propiedades y usos únicos.
La pureza del grafito de alta pureza es extremadamente alta, el contenido de impurezas es muy bajo, normalmente superior al 99,9%. Su excelente estabilidad química y alta conductividad eléctrica hacen que desempeñe un papel clave en la fabricación de semiconductores, la industria química de alta gama y otras industrias con estrictos requisitos de pureza.
El grafito prensado isostático se forma mediante un proceso de prensado isostático y tiene las características de estructura uniforme e isotropía. Tiene una excelente resistencia mecánica, resistencia a altas temperaturas y resistencia al choque térmico, un excelente rendimiento en metalurgia, electroerosión y otros campos.
El grafito expandido se fabrica a partir de grafito natural mediante un tratamiento especial, con una estructura única en forma de gusano. Puede expandirse rápidamente a altas temperaturas para formar un material con excelentes propiedades de aislamiento térmico y sellado. En el campo de los materiales ignífugos, el grafito expandido puede utilizarse para fabricar juntas ignífugas, revestimientos ignífugos, etc. Puede prevenir eficazmente la propagación del fuego.
¿De qué está hecho el grafito?
Elemento de grafito
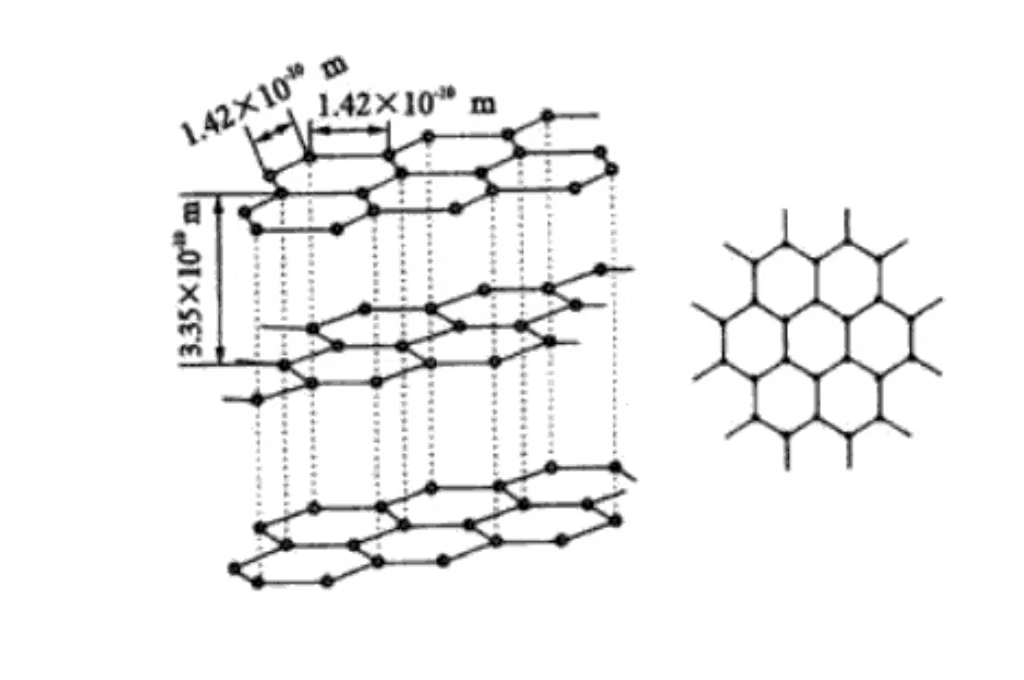
El principal elemento constitutivo del grafito es el carbono. En el grafito, los átomos de carbono están conectados entre sí por enlaces covalentes, formando una red plana de hexágonos. Estas estructuras de red planas se apilan capa sobre capa en el espacio, formando la estructura cristalina única del grafito. Cada átomo de carbono forma un enlace covalente con tres átomos de carbono circundantes. Y la presencia de este enlace covalente confiere al grafito una gran estabilidad y resistencia dentro de la capa. Al mismo tiempo, sienta las bases de algunas de sus propiedades especiales, como la conductividad eléctrica y térmica.
Fórmula química del grafito
La fórmula química del grafito suele denotarse por C, lo que indica claramente que está compuesto en su totalidad por carbono. A pesar de su sencilla fórmula química, el grafito presenta propiedades físicas y químicas complejas y diversas. Esto se debe a la disposición única de los átomos de carbono y a la existencia de numerosas formas de enlace químico. Esta característica, que se compone de un solo elemento pero tiene ricas propiedades, hace que el grafito ocupe una posición única en el campo de la ciencia de los materiales. Y también hace que se convierta en el centro de numerosas investigaciones y usos.
Estructura de grafito
Carbono Disposición de los átomos de carbono en el grafito
El carbono está dispuesto en la microestructura del grafito. Y los átomos de carbono muestran una notable característica de disposición en capas. Cada capa de átomos de carbono está estrechamente dispuesta para formar una enorme estructura de red plana hexagonal. Y los átomos de carbono están estrechamente conectados mediante enlaces covalentes. Esto hace que estas capas planas tengan una gran estabilidad y resistencia, y puedan soportar cierto grado de fuerza externa sin sufrir daños. La capa entre la capa es a través de la débil interacción de fuerza de van der Waals. Esta fuerza relativamente débil entre las capas hace que el grafito entre la capa y la capa cuando la pequeña fuerza externa es fácil de deslizar, dando así el grafito buena lubricación y flexibilidad. Por lo que necesita para reducir la fricción y tener una cierta capacidad de deformación en el escenario de aplicación.
Vinculación
El enlace covalente entre los átomos de carbono de la capa de grafito es un enlace químico fuerte. No sólo garantiza la estabilidad e integridad de la capa de grafito, sino que también tiene un profundo impacto en las propiedades físicas del grafito. Debido a la existencia de enlaces covalentes, los electrones pueden moverse con relativa libertad entre los átomos de carbono de la capa. Esto hace que el grafito tenga una buena conductividad y conductividad térmica dentro de la capa, y pueda transferir eficazmente la corriente y el calor.
La fuerza de van der Waals entre las capas es relativamente débil. Y su contribución a las propiedades físicas del grafito, como la dureza y la densidad, es menor que la de los enlaces covalentes. Este efecto sinérgico de los enlaces covalentes entre capas y las fuerzas de van der Waals entre capas crea las propiedades anisotrópicas únicas del grafito. Es decir, las propiedades físicas y químicas del grafito en la dirección de la capa y la capa vertical son significativamente diferentes. Y esta propiedad debe tenerse muy en cuenta en las aplicaciones de materiales para lograr un aprovechamiento óptimo de las propiedades del grafito.
Propiedades del grafito
Propiedades físicas
Color
El grafito suele presentar un color de apariencia gris-negra. La formación de este color está estrechamente relacionada con la estructura electrónica interna del grafito y sus características de absorción y reflexión de la luz. Los átomos de carbono del grafito absorben y dispersan la luz visible mediante enlaces químicos específicos y la distribución de la nube de electrones. De este modo, la mayor parte de la luz visible se absorbe y sólo una pequeña cantidad se refleja o dispersa. Así se presenta un efecto visual gris-negro a nivel macro. Además, la naturaleza opaca del grafito también confiere a su aspecto una textura única, en agudo contraste con otros materiales transparentes o translúcidos.
Densidad
La densidad del grafito es relativamente pequeña, entre 2,09-2,23 g/cm 3 aproximadamente. Y el peso específico también es bajo. Esta característica convierte al grafito en una clara ventaja en algunos escenarios de aplicación con estrictos requisitos de peso. Por ejemplo, en el diseño de ciertos componentes en el campo aeroespacial, si se necesita utilizar materiales que tengan cierto grado de propiedad conductora y lubricidad, pero que también puedan reducir el peso total. Entonces el grafeno se convierte en un material candidato muy potencial.
Punto de fusión
El grafito tiene un punto de fusión muy elevado, de unos 3652°C -3697 °C. Esta excelente estabilidad a altas temperaturas permite al grafito mantener su estructura y propiedades relativamente estables en entornos de temperaturas extremadamente altas. En la fundición de hierro y acero, los materiales refractarios y otros procesos industriales de alta temperatura, el grafito desempeña un papel vital.
Conductividad eléctrica
El grafito tiene una excelente conductividad eléctrica en la capa, que se debe a la formación de una estructura de nube de electrones estable entre los átomos de carbono de la capa mediante enlaces covalentes. Y los electrones pueden moverse con relativa libertad en esta estructura, para lograr una conducción eficaz de la corriente. Al mismo tiempo, la conductividad térmica del grafito también es muy buena, y puede transferir calor rápidamente.
Lubricación
Las propiedades lubricantes del grafito lubricante se deben a su singular estructura en capas. Debido a que la fuerza de van der Waals entre capas es débil. Cuando el grafito se somete a una fuerza externa, es fácil que se produzca el deslizamiento relativo entre las capas. Y este proceso de deslizamiento puede reducir eficazmente el coeficiente de fricción, para desempeñar un buen papel de lubricación. Tanto si se trata del mantenimiento diario de la lubricación de diversos equipos mecánicos en la industria de la maquinaria, como de las necesidades de lubricación en algunos entornos especiales (como alta temperatura, alta presión o entorno de corrosión química), el grafito puede mostrar excelentes efectos de lubricación.
Propiedades químicas
Resistencia a la corrosión del grafito
El grafito tiene una buena resistencia a los ácidos y álcalis. Puede mantener una estructura y un rendimiento relativamente estables en soluciones ácidas y alcalinas dentro de un determinado rango de concentración. Esto se debe a que los átomos de carbono del grafito forman una estructura de energía de enlace químico estable mediante enlaces covalentes. Esto hace que el grafito sea difícil de destruir por los iones de las soluciones ácidas y alcalinas. Esta resistencia a los ácidos y álcalis hace que el grafito tenga un importante valor de aplicación en algunos entornos corrosivos de la industria química.
Reactividad con otros materiales
En condiciones normales de temperatura, las propiedades químicas del grafito son relativamente estables. Y no es fácil que reaccione químicamente con la mayoría de las sustancias comunes. Sin embargo, cuando se expone a alta temperatura, alta presión o un entorno químico específico, el grafito puede reaccionar con algunos oxidantes (como el oxígeno, el ácido sulfúrico concentrado, etc.).
Por ejemplo, cuando el oxígeno es suficiente y la temperatura aumenta hasta cierto punto, el grafito sufrirá una reacción de oxidación y se transformará gradualmente en productos como el dióxido de carbono. Esta reactividad limita en cierta medida la aplicación del grafito en algunos entornos de oxidación extrema. Pero también ofrece la posibilidad de algunos tratamientos y modificaciones especiales del grafito.
Coeficiente de dilatación térmica
El grafito tiene un bajo coeficiente de dilatación térmica, lo que le confiere una buena estabilidad dimensional cuando cambia la temperatura. En comparación con muchos otros materiales, el volumen del grafito cambia muy poco durante el proceso de experimentar un mayor aumento y descenso de la temperatura.
En algunas aplicaciones que requieren una gran precisión dimensional de los materiales, este bajo coeficiente de expansión térmica del grafito es especialmente importante. Puede evitar eficazmente problemas como la deformación de los componentes y la reducción de la precisión de montaje causados por las fluctuaciones de temperatura. De este modo, se garantiza el funcionamiento normal y el rendimiento estable de los equipos o instrumentos en diferentes entornos de temperatura.
Oxidación
Aunque el grafito muestra una gran resistencia a la oxidación y la corrosión a temperatura ambiente, en condiciones extremas como alta temperatura, alta humedad o un entorno de fuerte oxidación, el grafito se oxidará y corroerá gradualmente. Por ejemplo, la exposición prolongada a altas temperaturas en el aire, los átomos de carbono de la superficie del grafito reaccionarán con el oxígeno para formar una capa de óxido.
Y con el tiempo, el continuo engrosamiento de la capa de óxido provocará cambios en la estructura y el rendimiento del grafito. Como la reducción de la conductividad y la resistencia. Por lo tanto, en algunas áreas de aplicación con altos requisitos de resistencia a la oxidación del grafito, a menudo es necesario llevar a cabo un tratamiento especial de la superficie del grafito. O añadir antioxidantes y otras medidas. Para mejorar su capacidad antioxidante con el fin de garantizar la estabilidad del rendimiento y la fiabilidad de los materiales de grafito durante su uso.
Propiedades mecánicas
Dureza y resistencia
La resistencia y dureza del grafito son relativamente bajas, y su dureza Mohs es de 1-2 aproximadamente. Esta propiedad hace que el grafito sea relativamente fácil de moldear y transformar en diversas formas durante su procesamiento. Por ejemplo, en la fabricación de minas para lápices, mezclando grafito y otros materiales como la arcilla en diferentes proporciones y prensándolos, es conveniente hacer minas para lápices con diferentes niveles de dureza para satisfacer diferentes necesidades de escritura. Sin embargo, aunque la resistencia general del grafito es baja, todavía tiene un cierto valor de utilización de la resistencia en algunas direcciones específicas. Por ejemplo, en la dirección de la capa de grafito.
Elasticidad
Debido a la singular estructura en capas del grafito, éste presenta cierta flexibilidad y elasticidad. Cuando se somete a una pequeña fuerza externa, podemos doblar y deformar la estructura en capas del grafito hasta cierto punto. Y cuando se retira la fuerza externa, podemos devolver al grafito su forma original o una forma cercana a la original. Esta flexibilidad y elasticidad hacen del grafeno una posible aplicación en algunos dispositivos electrónicos flexibles, materiales de sellado y otros campos emergentes.
Anisotropía
El grafito anisótropo presenta características anisótropas muy evidentes. Es decir, sus propiedades físicas y químicas son significativamente diferentes en distintas direcciones. En cuanto a la propiedad conductora, la conductividad a lo largo de la capa de grafito es mucho mayor que la conductividad en la capa vertical. Esto se debe al fomento de los enlaces covalentes en la conducción de electrones y al obstáculo de la fuerza de Van der Waals entre capas en la conducción de electrones.
En términos de dureza y resistencia, la dureza y la resistencia de la capa vertical son relativamente altas. Porque la fuerza de van der Waals entre las capas limita hasta cierto punto el deslizamiento relativo entre las capas. Mientras que el deslizamiento y la deformación son más probables debido a la débil fuerza entre capas. Esta característica anisotrópica requiere especial atención y consideración en el proceso de aplicación del grafito. Según los requisitos específicos de la aplicación, es razonable seleccionar y utilizar las ventajas de las propiedades del grafito en diferentes direcciones. De este modo, se maximiza la utilización del rendimiento del material de grafito y se optimiza el efecto de la aplicación.
Propiedades térmicas y eléctricas
Existe una estrecha relación interna entre las propiedades térmicas y eléctricas del grafito y sus propiedades térmicas y su excelente rendimiento. Su alta conductividad térmica es suficiente para emitir rápidamente el calor, lo que tiene un gran valor de aplicación en el campo de la disipación del calor de los equipos electrónicos. Al mismo tiempo, la buena conductividad del grafito le permite transmitir eficazmente la corriente como excelente conductor en el circuito.
Otras propiedades
Además de las muchas propiedades mencionadas anteriormente, el grafito tiene algunas otras propiedades especiales. Por ejemplo, el grafito tiene ciertas propiedades de adsorción. Su rica estructura porosa y su gran superficie específica pueden absorber algunos gases y moléculas pequeñas. Esta característica tiene un valor de aplicación potencial en la purificación de gases y el tratamiento de aguas residuales en el campo de la protección medioambiental. Mediante la modificación y el tratamiento adecuados del grafito, se puede mejorar aún más su capacidad de adsorción. Puede utilizarse para eliminar gases nocivos en el aire (como formaldehído, dióxido de azufre, etc.) o iones de metales pesados en el agua, contaminantes orgánicos, etc.
6 Usos del grafito
En Lápices
El grafito es un componente clave de la mina de los lápices. Gracias a su textura blanda y a su estructura única en capas, puede dejar marcas claras en el papel tras mezclarlo y ajustar su dureza con la arcilla para satisfacer las necesidades de la escritura y la pintura. Desde el aprendizaje de los estudiantes hasta la creación artística, se utiliza ampliamente en todo tipo de útiles de escritura. Permite expresar libremente las ideas y la creatividad.
Como lubricante
El grafito como lubricante tiene una buena lubricidad debido a la débil fuerza de Van der Waals en la capa intermedia y la capa intermedia de la estructura de capas. Se utiliza ampliamente en el campo de la mecánica. Ya sea en las piezas móviles internas del motor del automóvil o en las piezas de transmisión mecánica industrial. O incluso las piezas aeroespaciales de alta temperatura y alta presión, el polvo de grafito puede reducir eficazmente la fricción y el desgaste. Además, garantiza un funcionamiento suave y eficaz de los equipos y prolonga su vida útil.
Fabricación de acero
El grafito desempeña un papel importante en la fabricación del acero. Como electrodo, puede introducir corriente para producir la fusión por calor Joule de las materias primas de chatarra de acero. Como carburizador, se puede ajustar con precisión el contenido de carbono del acero fundido. Hecho de material de revestimiento del horno, en virtud de la resistencia a altas temperaturas y resistencia a la corrosión, proteger el cuerpo del horno de fabricación de acero de acero fundido a alta temperatura y los daños de escoria. Además, el grafito tiene una cierta capacidad calorífica específica. Puede absorber y liberar calor durante el proceso de fabricación del acero, ayudando a regular las fluctuaciones de temperatura en el horno. Apoya eficazmente el desarrollo eficiente, seguro y estable del proceso de fabricación de acero.
Batería
El grafito tiene una gran importancia en las baterías y se utiliza habitualmente como material de electrodos negativos en las baterías de iones de litio. pilas. Su estructura en capas proporciona espacio para la incrustación y desincrustación de iones de litio, la incrustación de carga, la descarga de descarga, con una buena propiedad conductora. Esto garantiza el ciclo de carga y descarga de la batería. En la investigación de la tecnología de baterías emergentes, también se considera el material básico. Esto desempeña un papel en la promoción del desarrollo del nuevo almacenamiento de energía.
Materiales refractarios
El grafito tiene un punto de fusión y una estabilidad de temperatura elevados, y es un material refractario de alta calidad. En metalurgia, cerámica, vidrio y otros procesos industriales de alta temperatura, se utiliza en la fabricación de ladrillos refractarios, revestimientos crisoles etc. Puede resistir la erosión del metal fundido y la escoria en entornos de alta temperatura, mantener la estabilidad estructural. Reducir la pérdida de calor, reducir el riesgo de accidentes, y construir una línea de seguridad fuerte para la producción industrial a alta temperatura.
Reactores nucleares
El grafito sirve de moderador de neutrones en los reactores nucleares. Al colisionar con los neutrones, los neutrones rápidos se ralentizan hasta convertirse en neutrones térmicos. De este modo, controlan la velocidad de la reacción de fisión nuclear y garantizan el funcionamiento estable del reactor. Los primeros reactores nucleares tienen muchas aplicaciones. Pero el grafito se altera con las altas temperaturas y la irradiación de neutrones, lo que exige respuestas técnicas especiales para garantizar un uso seguro a largo plazo.
Conclusión
Como alótropo del carbono, el grafito tiene diversas propiedades y se utiliza ampliamente. Sus diversas características están relacionadas entre sí, lo que determina su rendimiento en distintos escenarios. Desde los lápices de uso cotidiano hasta la siderurgia industrial y la fabricación de baterías, pasando por los reactores nucleares de alta tecnología, el grafito es indispensable. Con el desarrollo de la ciencia y la tecnología, el grafito tiene un gran potencial en campos emergentes. Y ocupará una posición más crítica en la ciencia de los materiales, la estrategia global de recursos y la estrategia de desarrollo sostenible. Y seguirá promoviendo el progreso de la sociedad humana.