Although they are both carbon, graphite and graphene could not be more different. They have a set of special characteristics that enables them to be likely used in various fields. In this article, you will learn about their structures and uses & costs. Let’s dive in.
Table of Contents
Toggle
Graphite vs Graphene
What is Graphite
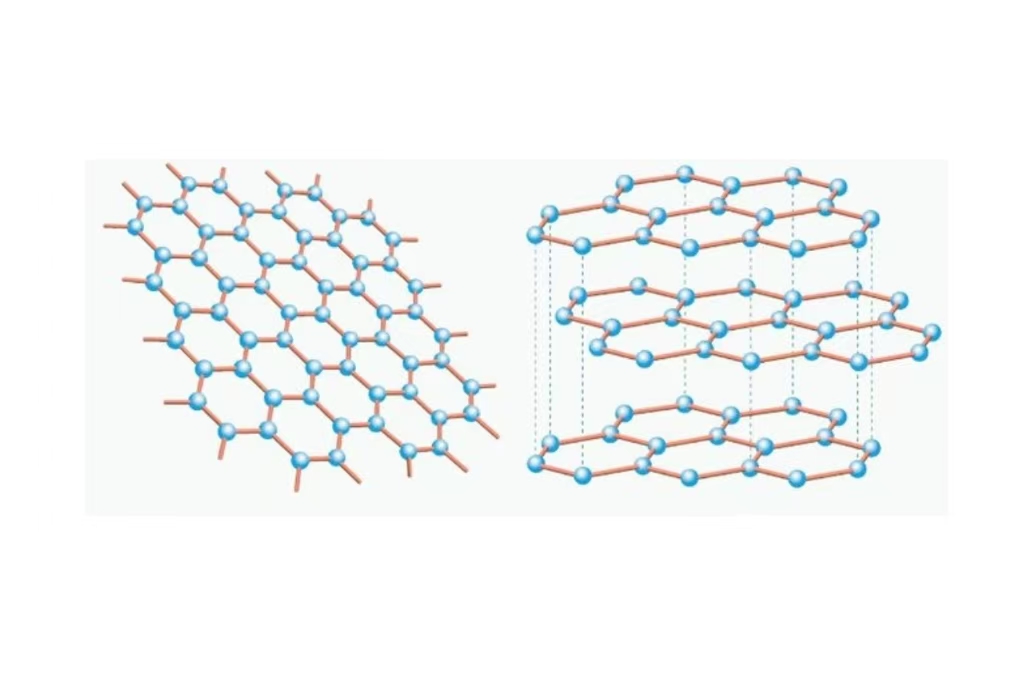
Graphite (a carbon allotrope) Sheet from carbon is hexagonal grid. This lubricious feel graphene take from their ability of layer sliding over each other. Graphite is now found within everyday items, from our pencils to lubricants and even batteries. It is still a conductor (though not as conductive as graphene, due to the fact that it is layered). Cheap and relatively abundant, it is a prime candidate for many industrial uses.
What is Graphene
Graphene is honeycomb arrangement of carbon atoms in a single layer. It is also single atom in thickness, so it means that the material itself can be super thin and still very strong. It is often referred to as the “miracle material” for its lightness and near-transparency, yet also because it is stronger than anything we have ever come across with a respectable electrical conductivity. That it is 200 times stronger than steel, and a single atom thick. The applications are numerous, from electronics to medical devices, and while the material is relatively abundant in nature creating it has been costly and difficult.
Structure
Layers vs Single Layer
Graphite: Consists of numerous layers of carbon sheets stacked over each other. As these layers weakly bind with each other, they can easily slide over one other. It is the reason we feel graphite in our hand as slippery and it also behaves like a lubricant.
Graphene: A single honeycombed layer of carbon atoms, a one atom thick material with exceptional strength and flexibility.
Bonding Differences
Graphite: Because the layers are held together by primitive van der Waals forces, graphite is easy to split or cleave.
Graphene: These carbon atoms are bonded very tightly within the single layer, which imbues graphene with its formidable strength and flexibility.
Properties of Graphite and Graphene
Electrical Conductivity
Graphite: While graphite also transfers electricity thanks to free moving electrons between its layers, those without additional graphene sheets here are not as conductive. The resistance arises when the layers are separated by a great enough distance.
Graphene: One of the best known electrical conductors Because electrons can coast through it with virtually no resistance, graphene is a natural for high-speed electronics.
Mechanical Strength
Graphite: Soft and brittle. Graphite snaps easily, hence it is used for pencil leads. Easy to become brittle as the layers are held together only by weak bonds.
Graphene: It is extremely tough, 20 times as robust as steel. Although only one carbon atom in thickness, the structure of graphene is due to its strong crystalline lattice and because each C-C bond has a strength equivalent to that of twice the length.
4.3Thermal Conductivity
Graphite: Decent thermal conductivity, thus see widespread use for example in passive cooling.
Graphene: Outstanding thermal conductivity. It is one of the best conductors for thermal energy, so it makes a good choice for cooling electrical components.
Applications
Graphite
Pencils: Pencils use graphite as the “lead” because it won’t smear or smudge too much, it will mark a surface easily and then erase when needed. Graphite consists of sheets in a layer, so they can write with very little pressure and leave a mark on paper.
Lubricants: As it is slippery, hence included in lubricants to prevent the friction among machinery. Since the layers of graphite are slick they allow them to slide easily over each other creating a solid barrier which reduces parts from wearing out due to movement.
Batteries: Graphite has also been found to be intrinsic element which can serve two primary functions within a lithium-ion battery anode; stability with efficient energy storage. This layered structure allows lithium ions to easily flow in and out during charge/discharge cycles.
Steelmaking: During steel manufacturing, it is generally applied as a refractory material because of withstanding high levels of heat. To do this, insulating linings in foundries and coating furnaces must maintain a constant temperature — an absolute requirement for the manufacture of high-quality steel.
Graphene
Electronics: Because graphene is an excellent conductor of electricity, one possibility suggested by two new experiments at Berkeley Lab and UC San Diego might be to use it in place of silicon in computer chips — allowing for faster devices that run cooler yet (ideally) consume less energy per computation. Its high electron mobility makes it ideally suited for the fast-switching speed needed in future computing technologies.
Medical Devices: Biocompatibility and sensitive grapheme make it ideal for biosensors, drug delivery system as well tissue engineering etc. This non-toxicity and compatibility with biological systems make it appealing for use in medical diagnostics or therapeutic treatments.
Energy Storage: Supercapacitors and advanced batteries could use graphene in the future to allow for a faster charge time with higher energy storage. Due to its high surface area and conductivity, the performance of energy storage devices can be improved, making them long lasting.
Composite Materials: Graphene can be combined with other materials such as metals and plastics to make them more lightweight while even stronger or electrically conductive. Potential applications of these graphene reinforced composites are to reduce weight, increase fuel efficiency and improve overall ablation performance.
Graphite vs Graphene: Cost
Graphite: Low
Abundance: Graphite is available inexpensively in common, natural form Mined worldwide, it is a readily available and cost-efficient mineral.
Production Costs:Due to its low production and processing costs, graphite is also suitable for use in many industrial applications.
Graphene: High
Production Challenges: Producing graphene is to significantly more difficult than producing graphite. Although monolayer h-BN can now be prepared using wet methods, existing fabrication approaches — such as chemical vapor deposition (CVD) and exfoliation techniques. It’s remain costly and are not easily scalable for large-scale manufacturing.
Price Trends: Despite the downward trend in graphene prices, cost for this material is much higher than that of graphite. Work is currently being conducted to create more cost-effective ways of producing graphene so that the material becomes commercially viable.
Conclusion
Graphite and graphene share some unique properties similar to each other which makes it an important group of materials. Accordingly, Therefore, we can consider graphene as the final step in the evolution of carbon. Graphite has served industries for decades, but Graphene is what many believe the real game changer which can disrupt a lot of fields across different sectors. If people can produce graphene more inexpensively and simply due to current research, a world where the other-worldly material is king might actually not be too far off. Whether you can respect graphite’s pragmatism or are seduced by graphene’s potential now. Both will have to work together moving forward in technology and industry.