Графит, являющийся одним из ключевых аллотропов углерода, играет важную роль во многих областях. Глубокое изучение его структуры - ключ к раскрытию широкого потенциала применения графита и разработке новых материалов.
Оглавление
Toggle
Что такое графит?
Графит, минерал, состоящий из атомов углерода, широко распространен в природе. Он имеет металлический блеск и на ощупь мягкий и гладкий. Это делает его идеальным материалом для грифелей карандашей. Цвет графита в основном черный или темно-серый. А его чистота и степень кристаллизации зависят от среды образования.
Атомная и молекулярная структура графита
Атомная структура графита
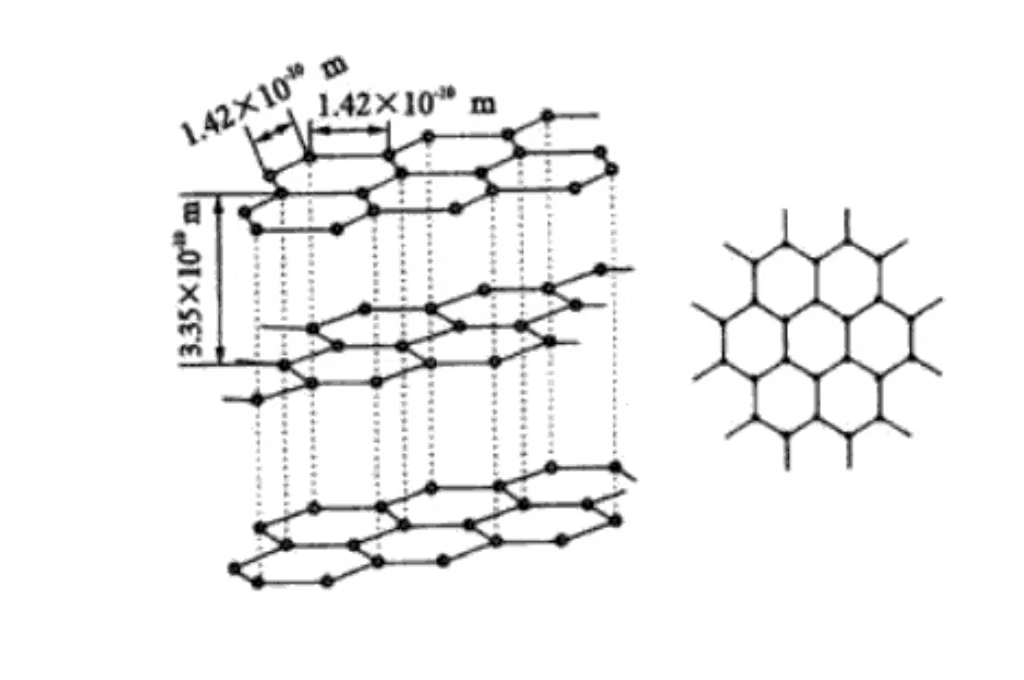
Основной состав графита - углерод. Атомы углерода в графите соединены ковалентными связями. При этом каждый атом углерода и окружающие его три атома углерода образуют устойчивую гексагональную кольцевую структуру, которая неограниченно расширяется в плоскости, образуя прочный атомный скелет.
Молекулярная структура графита
На молекулярном уровне графит состоит из слои атомов углерода, уложенных друг на друга. Межслоевые атомы углерода удерживаются относительно слабыми ван-дер-ваальсовыми силами. Такая слоистая структура объясняет, почему графит обладает превосходной смазывающей способностью и легко скользит между слоями.
Два ключевых элемента структуры графита
Гексагональная кристаллическая структура графита
Аранжировки
Графит имеет гексагональную кристаллическую структуру, атомы углерода тесно расположены в шестиугольниках в плоскости, в том числе под углом 120 градусов. Такое расположение является регулярным и стабильным, способствующим проводимости электронов, что является основой его хорошей электропроводности.
Многослойность
Атомы углерода уложены в параллельных плоскостях, расстояние между слоями составляет около 0,335 нм. Ван-дер-Ваальсовы силы между слоями слабые, поэтому графит легко скользит между внешними слоями и обладает смазываемостью. Он широко используется в качестве смазки в механическом производстве.
Слои кристаллической структуры
Каждый слой атомов углерода образует плоскость сети за счет ковалентных связей. Они упорядоченно расположены в пространстве, что придает графиту макроскопические кристаллические характеристики и анизотропию. Благодаря сильным ковалентным связям в слоях графит обладает высокой прочностью и твердостью в плоскости. В вертикальной плоскости прочность низкая из-за слабых межслоевых сил.
Связи между атомами углерода
Силы Ван дер Ваала
Межслоевые атомы углерода опираются на ван-дер-ваальсовы силы, которые слабы, что приводит к легкому скольжению между слоями графита и смазыванию. Однако при определенных условиях (например, при высокой температуре и давлении) межслойная структура графита становится изменчивой. Например, она может трансформироваться в алмазную структуру.
Разделение слоев
Благодаря слабым ван-дер-ваальсовым силам графитовый слой можно отделить, приложив небольшую сдвигающую силу. Это не только отражает смазывающую способность, но и создает возможность для реакций интеркаляции, благодаря которым физические и химические свойства графита могут быть изменены для получения специальных композиционных материалов. Например, материалов для отрицательных электродов литий-ионных аккумуляторов.
Ковалентные связи
Атомы углерода в слое плотно соединены ковалентными связями, образуя устойчивую гексагональную структуру. Это определяет высокую твердость и прочность графита в плоскости, гарантирует его структурную стабильность при применении в электродных материалах. А это ограничивает движение электронов, влияющих на анизотропию в плоскости.
Sp2 гибридизация
Угол сцепления
Атомы углерода принимают гибридизацию sp2, одна 2s и две 2p орбитали гибридизируются, образуя три эквивалентные орбитали гибридизации sp2. Они распределены в плоскости треугольника с углом около 120 градусов. Таким образом, атомы углерода образуют стабильные ковалентные связи с тремя соседними атомами углерода, образуя гексагональную структуру, которая способствует делокализации электронов и хорошей электропроводности.
Атомы углерода
Атом углерода образует плоский скелет с тремя окружающими атомами углерода через гибридные орбитали sp2. А вертикальные плоскости негибридных 2p-орбиталей перекрываются, образуя делокализованные π-электронные облака. π-электронные облака придают графиту хорошую электропроводность, при которой электроны могут свободно перемещаться в ответ на изменения электрических полей. А также делают графит активным в химических реакциях и участвуют в электрохимических процессах. Например, в качестве среды переноса электронов в литий-ионных батареях.
Анизотропия
Атрибуты в плоскости и атрибуты вне плоскости
Графит демонстрирует значительную анизотропию в различных направлениях. В плоскости ковалентная связь сильна, обладает высокой твердостью, прочностью и хорошей электропроводностью. Например, можно использовать композитные материалы, армированные графитовыми волокнами, в качестве армирующей фазы, чтобы использовать его прочность на растяжение в плоскости. В направлении вертикальной плоскости, из-за слабых межслойных ван-дер-ваальсовых сил, низкая прочность и плохая электропроводность. Эта характеристика делает его целенаправленным преимуществом в различных сценариях применения.
Договоренности по атомной энергии
Атомы углерода в графите расположены по определенному закону, образуя шестиугольники на плоскости и укладываясь слоями в пространстве. Такое расположение определяет кристаллическую структуру и физико-химические свойства. Рентгеновская дифракция позволяет определить степень кристалличности и структурные параметры по специфическому рисунку, представленному упорядоченным расположением. А стабильность атомного расположения позволяет графиту сохранять стабильные характеристики в определенном диапазоне температур и давлений. Например, графит как огнеупорный материал при высокой температуре может обеспечить целостность структуры, обеспечивая защиту для надежности промышленных применений.
Решетка и кристаллическая структура графита
Решетчатая структура графита
Графит имеет гексагональную решетчатую структуру, оси a и b имеют одинаковую длину. А угол составляет 120 градусов, ось c перпендикулярна плоскости атома углерода. Ее длина отражает периодическое расположение слоистой структуры, относится к гексагональной кристаллической системе, с определенной симметрией и кристаллографическими характеристиками.
Кристаллическая структура графита
Кристалл графита состоит из многочисленных единиц гексагональной решетки, упорядоченно расположенных в пространстве. А внутренние атомы углерода расположены в высшей степени упорядоченно. Дефекты и примеси в нем значительно изменяют его характеристики, влияя на перенос электронов и фононов, химические реакции и однородность материала.
Три распространенных дефекта в структуре графита
Дефекты в структуре графита оказывают большое влияние на его эксплуатационные характеристики.
Точечные дефекты, такие как вакансии и зазоры между атомами, разрушают целостность атомов, влияют на проводимость электронов и механические свойства.
Линейные дефекты, такие как дислокации, влияют на пластическую деформацию и прочность.
Дефекты поверхности, такие как границы зерен, препятствуют передаче электронов и фононов, снижают электропроводность и теплопроводность. А это легко приводит к химическим реакциям и скоплению примесей.
Концепции, связанные со структурой графита
Структура Льюиса графита
Структура Льюиса графита демонстрирует обмен электронами между атомами углерода и удовлетворяет восьмиэлектронной стабильной структуре, образуя ковалентные связи с соседними атомами углерода. Незадействованные электроны образуют делокализованные π-электронные облака. Это дает основу для понимания химической связи и распределения электронов.
Гибридизация графита
Гибридизация sp2 атомов углерода графита лежит в основе его уникальной структуры и свойств. В результате образуется планарная структура, делокализованное облако π-электронов, что придает графиту целый ряд превосходных свойств.
Графитовые символы и формулы
Химический символ графита - "C". Хотя макромолекулярную структуру трудно выразить простой молекулярной формулой, но в химических расчетах и формулах реакций. "C" может представлять реакцию графита, отражая трансформацию и сохранение углерода.
Структура и связь графита
Слоистая структура и свойства поверхности графита имеют большое значение для его связующих свойств. Межслойные ван-дер-ваальсовы силы слабы, поэтому для усиления взаимодействия необходимо модифицировать поверхность графита или выбрать подходящее связующее. Модификация поверхности может включать в себя введение функциональных групп или обработку огрублением. А полярные группы связующего могут прочно связываться с атомами углерода на поверхности графита. В композитных материалах хорошее сцепление является ключевым фактором, гарантирующим общие механические и функциональные свойства. А плохое скрепление легко может привести к концентрации межфазных напряжений, что приведет к разрушению материала.
Объясните строение графита и различие других материалов
Структура графита и структура графена
На самом деле, графен представляет собой слой графита толщиной в один атом. В каждом листе графита толщиной 1 мм содержится около 3 миллионов слоев графена, уложенных друг на друга. Графен можно рассматривать как один слой графита, в то время как графит состоит из нескольких слоев графена, расположенных друг над другом.
Сравнение структуры графита и алмаза
Структурные различия
Атомы углерода в алмазе имеют гибридную структуру sp3, образуя тетраэдрическую пространственную структуру, а ковалентные связи между атомами очень сильны. Графит имеет sp2-гибридную планарно-гексагональную и слоистую структуру, со слабыми ван-дер-ваальсовыми силами между слоями.
Разница в производительности
Структурные различия приводят к явным различиям в эксплуатационных характеристиках. Твердость алмаза очень высока, используется в механической обработке; графит имеет мягкую текстуру, хорошую смазывающую способность, используется в качестве смазки и грифеля для карандашей. Графит проводит электричество, алмаз - с трудом. Алмаз имеет высокий коэффициент преломления и прозрачность, используется в ювелирных изделиях; графит черный и непрозрачный.
Типы структуры графита
Натуральный графит
Обычно он встречается в графитовых сланцах, графитовых гнейсах, графитоносных сланцах и метаморфических сланцах. По кристаллической форме, природный графит одновременно можно разделить на две разновидности: кристаллический графит, который далее подразделяется на чешуйчатый графит, и криптокристаллический графит, также известный как земляной графит.
Синтетический графит
Синтетический графит это вид химической продукции. Его основным ингредиентом является углерод. Его получают путем высокотемпературного пиролиза и графитизации органических полимеров.
Специальные графитовые структуры, такие как расширяемый графит и нанографитовые структуры. Расширяющийся графит путем специальной обработки, межслойный материал вставки, разложение и расширение при высокой температуре, с хорошим антипиреном, используется для огнезащитных материалов. Нанографитовые структуры, такие как нанографитовые листы и нанографитовые волокна, имеют большую удельную поверхность, высокую поверхностную активность и отличные механические свойства. Они имеют большой потенциал в области хранения энергии, носителей катализаторов и высокоэффективных композитных материалов.
Взаимосвязь между структурой графита и его использованием
Уникальная структура графита обуславливает его широкое применение. Хорошая электропроводность делает его электродным материалом, который используется в аккумуляторах и электролитических элементах. Высокая температурная стабильность и химическая инертность делают его огнеупором для сталелитейной промышленности. Смазочные свойства позволяют использовать его в качестве смазки в машиностроении. В аэрокосмической отрасли графитовые композиты используются при производстве компонентов самолетов и ракет благодаря низкой плотности, высокой прочности и термостойкости. Кроме того, графит играет важную роль в производстве карандашей, подготовке графена и других областях. И каждое из этих применений тесно связано со структурой графита.
Заключение
Структура графита демонстрирует свою уникальность и сложность в нескольких измерениях, что оказывает глубокое влияние на его характеристики и применение. Глубокое изучение и понимание структуры графита открывает широкие перспективы для его инновационного применения во многих областях, таких как материаловедение и энергетика. А это поможет преодолеть материальные и энергетические проблемы современного общества.