Le graphite, allotrope clé du carbone, joue un rôle important dans de nombreux domaines. L'exploration approfondie de sa structure est la clé qui permettra d'exploiter le vaste potentiel d'application du graphite et de développer de nouveaux matériaux.
Table des matières
Toggle
Qu'est-ce que le graphite ?
Le graphite, un minéral composé d'atomes de carbone, est largement répandu dans la nature. Il présente un éclat métallique et un toucher doux et lisse. Il s'agit donc d'un matériau idéal pour les mines de crayon. La couleur du graphite est généralement noire ou gris foncé. Sa pureté et son degré de cristallisation varient en fonction de l'environnement de formation.
Structure atomique et moléculaire du graphite
Structure atomique du graphite
Le graphite est principalement composé de carbone. Les atomes de carbone du graphite sont reliés par des liaisons covalentes. Chaque atome de carbone et les trois atomes de carbone qui l'entourent forment une structure stable d'anneau hexagonal, qui s'étend indéfiniment dans le plan pour former un squelette atomique solide.
Structure moléculaire du graphite
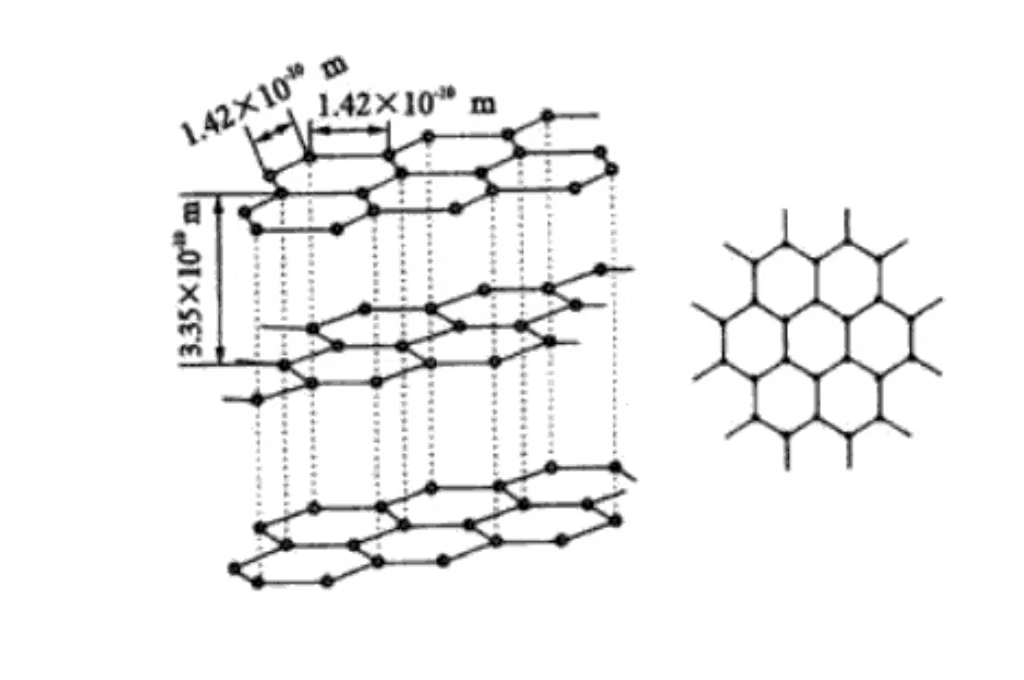
Au niveau moléculaire, le graphite est composé de couches d'atomes de carbone empilés les uns sur les autres. Les atomes de carbone entre les couches sont maintenus par des forces de van der Waals relativement faibles. Cette structure en couches explique l'excellent pouvoir lubrifiant du graphite et la facilité de glissement entre les couches.
Deux éléments clés de la structure du graphite
Structure cristalline hexagonale du graphite
Arrangements
Le graphite a une structure cristalline hexagonale, les atomes de carbone sont étroitement disposés en hexagones dans le plan, y compris un angle de 120 degrés. La disposition est régulière et stable, propice à la conduction des électrons, ce qui est à la base de sa bonne conductivité électrique.
Superposition
Les atomes de carbone sont empilés dans des plans parallèles, l'espacement des couches est d'environ 0,335 nm. La force de van der Waals entre les couches est faible, ce qui permet au graphite de glisser facilement entre les couches externes et de se lubrifier. Il est couramment utilisé comme lubrifiant dans le domaine de la fabrication mécanique.
Couches de la structure cristalline
Chaque couche d'atomes de carbone forme un plan de réseau par le biais de liaisons covalentes. Cette disposition ordonnée dans l'espace confère au graphite ses caractéristiques cristallines macroscopiques et son anisotropie. La forte liaison covalente dans la couche confère au graphite une résistance et une dureté élevées dans le plan. La direction du plan vertical a une faible résistance en raison de la faible force entre les couches.
Liaisons entre atomes de carbone
Forces Van der Waal
Les atomes de carbone entre les couches s'appuient sur la force de van der Waals, qui est faible, ce qui facilite la séparation par glissement entre les couches de graphite et le pouvoir lubrifiant. Mais cela rend également la structure intercalaire du graphite variable dans certaines conditions (telles que la température et la pression élevées). Elle peut ainsi se transformer en une structure de diamant.
Séparation des couches
En raison de la faible force de van der Waals, la couche de graphite peut être séparée en appliquant une petite force de cisaillement. Cela ne reflète pas seulement le pouvoir lubrifiant, mais crée également la possibilité de réactions d'intercalation, par lesquelles les propriétés physiques et chimiques du graphite peuvent être modifiées pour préparer des matériaux composites spéciaux. Tels que les matériaux d'électrodes négatives pour les batteries lithium-ion.
Liaisons covalentes
Les atomes de carbone de la couche sont étroitement liés par des liaisons covalentes pour former une structure hexagonale stable. Cela détermine la dureté et la résistance élevées du graphite dans le plan, et garantit sa stabilité structurelle dans l'application des matériaux d'électrodes. Elle limite également le mouvement des électrons, ce qui affecte l'anisotropie dans le plan.
Hybridation Sp2
Angle de collage
Les atomes de carbone adoptent l'hybridation sp2, une orbitale 2s et deux orbitales 2p s'hybrident pour former trois orbitales d'hybridation sp2 équivalentes. Celles-ci sont réparties dans un triangle plan dont l'angle est d'environ 120 degrés. Ainsi, les atomes de carbone forment des liaisons covalentes stables avec trois atomes de carbone adjacents pour construire une structure hexagonale, qui est propice à la conduction par délocalisation des électrons et à une bonne conductivité électrique.
Atomes de carbone
L'atome de carbone construit un squelette planaire avec trois atomes de carbone environnants par le biais d'orbitales hybrides sp2. Les plans verticaux des orbitales 2p non hybrides se chevauchent pour former des nuages d'électrons π délocalisés. Les nuages d'électrons π confèrent au graphite une bonne conductivité électrique, dans laquelle les électrons peuvent se déplacer librement en réponse à des changements de champs électriques. Ils rendent le graphite actif dans les réactions chimiques et participent aux processus électrochimiques. Il sert par exemple de support de transfert d'électrons dans les batteries lithium-ion.
Anisotropie
Attributs dans le plan et attributs hors du plan
Le graphite présente une anisotropie significative dans différentes directions. Dans le plan, la liaison covalente est forte, avec une dureté et une résistance élevées et une bonne conductivité électrique. On peut ainsi utiliser des matériaux composites renforcés par des fibres de graphite comme phase de renforcement pour utiliser sa résistance à la traction dans le plan. Dans la direction du plan vertical, en raison de la faible force de van der Waals entre les couches, la résistance est faible et la conductivité médiocre. Cette caractéristique lui confère un avantage ciblé dans différents scénarios d'application.
Dispositions relatives à l'énergie atomique
Les atomes de carbone du graphite sont disposés selon une loi spécifique, formant des hexagones dans le plan et des couches empilées dans l'espace. Cette disposition détermine la structure cristalline et les propriétés physiques et chimiques. La diffraction des rayons X permet de déterminer la cristallinité et les paramètres structurels en fonction du modèle spécifique présenté par son arrangement ordonné. La stabilité de l'arrangement atomique permet au graphite de conserver des performances stables dans une certaine plage de température et de pression. Le graphite en tant que matériau réfractaire à haute température peut garantir l'intégrité de la structure, assurant ainsi la protection de la fiabilité des applications industrielles.
Réseau et structure cristalline du graphite
Structure du réseau du graphite
Le graphite a une structure hexagonale, les axes a et b sont de même longueur. L'angle est de 120 degrés, l'axe c est perpendiculaire au plan de l'atome de carbone. Sa longueur reflète l'arrangement périodique de la structure en couches, appartenant au système cristallin hexagonal, avec une symétrie et des caractéristiques cristallographiques spécifiques.
Structure cristalline du graphite
Le cristal de graphite est constitué de nombreuses unités de réseau hexagonales disposées de manière ordonnée dans l'espace. Les atomes de carbone internes sont disposés de manière très ordonnée. Les défauts et les impuretés modifient considérablement ses performances, en affectant le transport des électrons et des phonons, les réactions chimiques et l'uniformité du matériau.
Trois défauts courants dans la structure du graphite
Les défauts dans la structure du graphite ont une grande influence sur ses performances.
Les défauts ponctuels, tels que les atomes vacants et les atomes de dégagement, détruisent l'intégrité atomique et affectent la conduction des électrons et les propriétés mécaniques.
Les défauts linéaires tels que les dislocations affectent la déformation plastique et la résistance.
Les défauts de surface, tels que les joints de grains, entravent la transmission des électrons et des phonons, réduisent la conductivité et la conductivité thermique. De plus, ils entraînent facilement des réactions chimiques et l'agrégation d'impuretés.
Concepts liés à la structure du graphite
Structure de Lewis du graphite
La structure de Lewis du graphite montre le partage des électrons entre les atomes de carbone et satisfait à la structure stable à huit électrons en formant des liaisons covalentes avec les atomes de carbone voisins. Les électrons non impliqués forment des nuages d'électrons π délocalisés. Cela permet de comprendre la liaison chimique et la distribution des électrons.
Hybridation du graphite
L'hybridation sp2 des atomes de carbone du graphite est à l'origine de sa structure et de ses propriétés uniques. Il en résulte une structure planaire, un nuage d'électrons π délocalisé, qui confère au graphite une variété d'excellentes propriétés.
Symboles et formules de graphite
Le symbole chimique du graphite est "C". Bien qu'il soit difficile d'exprimer la structure macromoléculaire par une simple formule moléculaire, dans le calcul chimique et la formule de réaction, "C" peut représenter la réaction du graphite, reflétant la transformation et la conservation du carbone. "C" peut représenter la réaction du graphite, reflétant la transformation et la conservation du carbone.
Structure et liaison du graphite
La structure en couches et les propriétés de surface du graphite sont d'une grande importance pour ses propriétés de liaison. La force de van der Waals entre les couches étant faible, il est nécessaire de modifier la surface du graphite ou de choisir un liant approprié pour renforcer l'interaction. La modification de la surface peut se faire par l'introduction de groupes fonctionnels ou par un traitement d'épaississement. Les groupes polaires du liant peuvent se lier fortement aux atomes de carbone de la surface du graphite. Dans les matériaux composites, une bonne performance de liaison est essentielle pour garantir les propriétés mécaniques et fonctionnelles globales. Une mauvaise liaison peut facilement provoquer une concentration de contraintes interfaciales, entraînant une défaillance du matériau.
Expliquer la structure du graphite et d'autres matériaux Différence
Structure du graphite et structure du graphène
En fait, graphène représente une couche de graphite d'un atome d'épaisseur. Chaque feuille de graphite de 1 mm d'épaisseur contient environ 3 millions de couches de graphène empilées les unes sur les autres. Le graphène peut être considéré comme une couche de graphite, tandis que le graphite est constitué de plusieurs couches de graphène superposées.
Comparaison de la structure du graphite et du diamant
Différences structurelles
Les atomes de carbone du diamant adoptent une hybridation sp3 pour former une structure spatiale tétraédrique, et les liaisons covalentes entre les atomes sont très fortes. Le graphite est un hybride sp2 planaire hexagonal et stratifié, avec de faibles forces de van der Waals entre les couches.
Différences de performance
Les différences structurelles se traduisent par des différences de performance distinctes. La dureté du diamant est très élevée, il est utilisé pour l'usinage ; le graphite a une texture douce, un bon pouvoir lubrifiant, il est utilisé comme lubrifiant et comme mine de crayon. Le graphite conduit l'électricité, le diamant difficilement. Le diamant a un indice de réfraction et une transparence élevés, il est utilisé en bijouterie ; le graphite est noir et opaque.
Types de structure du graphite
Graphite naturel
Il est généralement présent dans les schistes graphiteux, les gneiss graphiteux, les schistes graphiteux et les schistes métamorphiques. Selon la forme cristalline, graphite naturel peut être divisé simultanément en deux variétés : le graphite cristallin, qui se subdivise à son tour en graphite lamellaire, et le graphite cryptocristallin, également connu sous le nom de graphite terreux.
Graphite synthétique
Graphite synthétique est une sorte de produit chimique. Son principal ingrédient est le carbone. Il est obtenu par pyrolyse à haute température et graphitisation de polymères organiques.
Structures de graphite spéciales, telles que le graphite expansible et les structures de nanographite. Le graphite expansible est soumis à un traitement spécial, un matériau d'insertion intercalaire, une décomposition et une expansion à haute température, un bon retardateur de flamme, utilisé pour les matériaux ignifuges. Les structures de nano-graphite, telles que les feuilles et les fibres de nano-graphite, ont une grande surface spécifique, une activité de surface élevée et d'excellentes propriétés mécaniques. Elles présentent un grand potentiel dans les domaines du stockage de l'énergie, des supports de catalyseurs et des matériaux composites à haute performance.
Corrélation entre la structure du graphite et son utilisation
La structure unique du graphite détermine sa large utilisation. Sa bonne conductivité électrique en fait un matériau d'électrode, utilisé dans les batteries et les cellules électrolytiques. Sa stabilité à haute température et son inertie chimique en font un réfractaire pour l'industrie sidérurgique. Ses propriétés lubrifiantes lui permettent d'être utilisé comme lubrifiant dans la fabrication de machines. Dans le domaine aérospatial, les composites à base de graphite sont utilisés dans la fabrication de composants d'avions et de fusées en raison de leur faible densité, de leur résistance élevée et de leur stabilité thermique. En outre, le graphite joue également un rôle important dans la fabrication des crayons, la préparation du graphène et d'autres domaines. Chaque application est étroitement liée à la structure du graphite.
Conclusion
La structure du graphite montre son caractère unique et sa complexité dans de multiples dimensions, ce qui affecte profondément ses performances et ses applications. La recherche approfondie et la compréhension de la structure du graphite ouvrent de vastes perspectives pour son application innovante dans de nombreux domaines tels que la science des matériaux et l'énergie. Cela permet de résoudre les problèmes de matériaux et d'énergie de la société moderne.